Abstracts
PURPOSE: To verify the effect of highly concentrated platelet-rich plasma (hPRP) in the pathways of bone repair using non-critical defects in the calvaria of rabbits. METHODS: The hPRP was produced from collected venous blood of 21 rabbits. Four non-critical defects of 8 mm in diameter were created on the calvaria of each animal. The defects were all treated differently: autogenous particled bone (APB, group 1), autogenous particled bone associated with hPRP (APB + hPRP, group 2), isolated hPRP (group 3), and blood clot (control, group 4). Animals were submitted to euthanasia on the 2nd, 4th and 6th week postoperatively. Histological and histomorphometric analysis were carried through. RESULTS: After two weeks, groups 1 and 2 were in more advanced stage of repair than 3 and 4. At this period, comparing the groups 1 and 2, no significant differences were found between both, which also happened between the groups 3 and 4. However, after four and six weeks, the group 1 showed a more advanced stage of repair among all the other studied groups, while group 2 was in more advanced signs of bone repair than groups 3 and 4. Comparing groups 3 and 4, after four and six weeks, the least advanced stage of bone repair was found to be within group 3. CONCLUSION: The use of a highly concentrated PRP was considered prejudicial to the repair of non-critical defects in the calvaria of rabbits, either in the association of autogenous particled bone, when compared to autogenous particled bone alone, or in its isolated form, when compared to blood clot (control).
BoneTransplantation; Bone Regeneration; Platelet-Rich, Plasma; Skull; Rabbits
OBJETIVO: Verificar os efeitos do plasma rico em plaquetas altamente concentrado (hPRP) sobre o reparo ósseo, utilizando defeitos não críticos na calvária de coelhos. MÉTODOS: O concentrado de plaquetas foi produzido a partir de sangue venoso coletado de 21 coelhos. Quatro defeitos não críticos de 8 mm de diâmetro foram criados na calvária de cada animal. Os defeitos foram tratados de modo distinto: osso autógeno particulado (grupo 1), osso autógeno particulado associado com hPRP (grupo 2), hPRP de modo isolado (grupo 3) e coágulo sangüineo (controle, grupo 4). Os animais foram mortos na 2º, 4º e 6º semanas do pós-operatório. Análises histológicas e histomorfométricas foram realizadas. RESULTADOS: Em duas semanas, os grupos 1 e 2 estavam num estado de reparação mais adiantado que os grupos 3 e 4. Neste período, quando comparados os grupos 1 e 2, não foram observadas diferenças estatísticamente significativas, o mesmo acontecendo quando a comparação foi entre os grupos 3 e 4. Após quatro e seis semanas, contudo, o grupo1 mostrou um estágio mais avançado de reparo, isto quando comparado com todos os outros grupos estudados, enquanto o grupo 2 apresentou sinais mais avançados de reparo que os grupos 3 e 4. Comparando os grupos 3 e 4, após 4 e 6 semanas, um estágio menos avançado do reparo ósseo foi observado no grupo 3. CONCLUSÃO: O uso do plasma rico em plaquetas altamente concentrado foi considerado prejudicial ao reparo de defeitos não críticos na calvária de coelhos, tanto quando em associação com enxerto ósseo autógeno em partículas (quando comparado com enxerto ósseo em partículas de forma isolada) quanto em sua forma isolada (quando comparado com o coágulo sangüíneo-controle).
Transplante Ósseo; Regeneração Óssea; Plasma Rico em Plaquetas; Crânio; Coelhos
7 - ORIGINAL ARTICLE
MODELS, BIOLOGICAL
Effects of a highly concentrated platelet-rich plasma on the bone repair using non-critical defects in the calvaria of rabbits1 1 Research performed at the Principles of Surgery Post-Graduate Program, Evangelical Medical School, Curitiba-PR, Brazil.
Efeitos do plasma rico em plaquetas altamente concentrado no reparo ósseo, utilizando defeitos não-críticos na calvária de coelhos
Marco Antonio de Oliveira FilhoI; Paulo Afonso Nunes NassifII; Osvaldo MalafaiaII; Jurandir Marcondes Ribas FilhoII; Carmen Austrália Paredes Marcondes RibasII; Alex Coronel CamachoIII; Edmar Stieven FilhoIV; Allan Fernando GiovaniniV
IPhD, Principles of Surgery Post-Graduate Program, Evangelical Medical School, Curitiba-PR, Brazil
IIPhD, Full Professor, Post-Graduate Program, Evangelical Medical School, Curitiba-PR, Brazil
IIIMaster Post-Graduate Program, Evangelical Medical School, Curitiba-PR, Brazil
IVMaster Degree student, Post-Graduate Program, Evangelical Medical School, Curitiba-PR, Brazil
VPhD, Oral Pathology, Sao Paulo University (USP), Brazil
Correspondence Correspondence: Marco Antonio de Oliveira Filho R. Padre Anchieta, 2454/304 80730-000 Curitiba - PR Brazil Phone: (55 41)3240-5488 drmarcoantonio@uol.com.br
ABSTRACT
PURPOSE: To verify the effect of highly concentrated platelet-rich plasma (hPRP) in the pathways of bone repair using non-critical defects in the calvaria of rabbits.
METHODS: The hPRP was produced from collected venous blood of 21 rabbits. Four non-critical defects of 8 mm in diameter were created on the calvaria of each animal. The defects were all treated differently: autogenous particled bone (APB, group 1), autogenous particled bone associated with hPRP (APB + hPRP, group 2), isolated hPRP (group 3), and blood clot (control, group 4). Animals were submitted to euthanasia on the 2nd, 4th and 6th week postoperatively. Histological and histomorphometric analysis were carried through.
RESULTS: After two weeks, groups 1 and 2 were in more advanced stage of repair than 3 and 4. At this period, comparing the groups 1 and 2, no significant differences were found between both, which also happened between the groups 3 and 4. However, after four and six weeks, the group 1 showed a more advanced stage of repair among all the other studied groups, while group 2 was in more advanced signs of bone repair than groups 3 and 4. Comparing groups 3 and 4, after four and six weeks, the least advanced stage of bone repair was found to be within group 3.
CONCLUSION: The use of a highly concentrated PRP was considered prejudicial to the repair of non-critical defects in the calvaria of rabbits, either in the association of autogenous particled bone, when compared to autogenous particled bone alone, or in its isolated form, when compared to blood clot (control).
Key words: BoneTransplantation. Bone Regeneration. Platelet-Rich, Plasma. Skull. Rabbits.
RESUMO
OBJETIVO: Verificar os efeitos do plasma rico em plaquetas altamente concentrado (hPRP) sobre o reparo ósseo, utilizando defeitos não críticos na calvária de coelhos.
MÉTODOS: O concentrado de plaquetas foi produzido a partir de sangue venoso coletado de 21 coelhos. Quatro defeitos não críticos de 8 mm de diâmetro foram criados na calvária de cada animal. Os defeitos foram tratados de modo distinto: osso autógeno particulado (grupo 1), osso autógeno particulado associado com hPRP (grupo 2), hPRP de modo isolado (grupo 3) e coágulo sangüineo (controle, grupo 4). Os animais foram mortos na 2º, 4º e 6º semanas do pós-operatório. Análises histológicas e histomorfométricas foram realizadas.
RESULTADOS: Em duas semanas, os grupos 1 e 2 estavam num estado de reparação mais adiantado que os grupos 3 e 4. Neste período, quando comparados os grupos 1 e 2, não foram observadas diferenças estatísticamente significativas, o mesmo acontecendo quando a comparação foi entre os grupos 3 e 4. Após quatro e seis semanas, contudo, o grupo1 mostrou um estágio mais avançado de reparo, isto quando comparado com todos os outros grupos estudados, enquanto o grupo 2 apresentou sinais mais avançados de reparo que os grupos 3 e 4. Comparando os grupos 3 e 4, após 4 e 6 semanas, um estágio menos avançado do reparo ósseo foi observado no grupo 3.
CONCLUSÃO: O uso do plasma rico em plaquetas altamente concentrado foi considerado prejudicial ao reparo de defeitos não críticos na calvária de coelhos, tanto quando em associação com enxerto ósseo autógeno em partículas (quando comparado com enxerto ósseo em partículas de forma isolada) quanto em sua forma isolada (quando comparado com o coágulo sangüíneo-controle).
Descritores: Transplante Ósseo. Regeneração Óssea. Plasma Rico em Plaquetas. Crânio. Coelhos.
Introduction
Multiple uncertainties still exist about the action of the growth factors and the platelet-rich plasma and its effect on the tissue repair. It is known that platelets are a source of several growth factors, among which are; PDGF1-4, TGF-b5,6, VEGF7,8, IGF-I4,9 and EGF6,10. This fact stimulated the development of a platelet concentrate with the intention of increasing the levels of local growth factors delivery, which, theoretically, if present at a damaged site, could improve the healing process.
Several authors started to use the platelet-rich plasma (PRP), mainly in association with autogenous bone graftings4,7,9,12, and also to improve soft tissue repair4,10.
Although there is still no consensus about the ideal platelet concentration that could optimize the tissue repair process, some in vivo and in vitro studies suggest that a PRP highly concentrated could even be harmful to the repair13-15.
Some studies show positive PRP results2,7 while others present poor PRP results5. In PRP experimentation there is a concern about the employed method of concentration regarding the ability to really concentrate platelets. It is also important not to damage the platelets during the process10. Thus, more studies in vivo and in vitro may contribute to clarify aspects of PRP use and its effectiveness.
The present study aims to analyse the effects of a highly concentrated PRP (hPRP) on the mechanism of tissue repair of non-crictical defects in the calvaria of rabbits.
Methods
Animal model and PRP preparation
This study was performed at the Institute of Medical Research (IPEM) of the Evangelic University Hospital of Curitiba, according to its Committee of Ethics. All animals used in the designed study were from the IPEM laboratory. Twenty one white rabbits (New Zealand), female, age between 350-370 days, weight of 2,750-4,600g, with no previous disease, were used.
Animals were anesthetized with an intramuscular injection of ketamine 5% (0,5mL/Kg). For the collection of venous blood the most favorable ear vein of each animal was punctioned, using a scalp 21. Afterwards, a seringe of 20 mL with 10% sodium citrate was connected to the scalp.
Approximately 15 mL of blood of each rabbit was collected; 10 mL were transferred to a tube 16 x 100 mm and 4 mL to other tube 12 x 75 mm. Automatic platelet counting was done using the blood of the 12 x 75 mm tube.
For the preparation of the hPRP a regular centriphuge was used (Excelsa Baby II 206-R). At first, tubes were placed on 200g for 20 minutes, allowing the formation of two distinct fractions; plasma at the superior part of the tube (slightly yellow colored) and the blood cells fraction at the bottom (red colored). All of the plasma fraction plus the upper (1 mL) part of the blood cells fraction were transferred to another tube, submitted to a second cycle of 400g for 10 minutes. After this last cycle, two distinct fractions could be identified. The upper fraction (yellow colored) was removed to the point where its remanescent plus the bottom fraction (red colored) completed a total of 1 mL. After homogeneization, 1 mL of final product - from the initial 10 mL of blood was obtained. Another automatic counting of platelet was done.
Surgical procedure
The region was previously shaved and asseptically prepared. Sterile barriers limited surgical field. The region was injected sub-periosteally with 1 mL of lidocaine 2% with adrenaline 1:100.000. A midline dermo-periosteal incision (5 cm) was made, raising a skin-periosteal flap to expose the calvarial surface. Four defects of 8 mm in diameter were created with a trephine under profuse saline solution irrigation. Bone fragments were particled for grafting. For the hPRP coagulation, a mixture of 10% calcium chloride solution and 5000 units of bovine thrombin were added to the previously prepared hPRP. (1 minute for gel obtaintion). One defect was grafted with autogenous particled bone (APB, group 1), another with autogenous particled bone associated with hPRP (APB + hPRP, group 2), isolated hPRP (group 3), and no grafting (control, group 4) (Figure 1, Table 1). Tissue flaps of the wound were ininterrupted sutured.
Histologic and histomorphometric assessments
The animals were dead with an overdose of the anaesthetic solution after two weeks (7 animals), 4 weeks (7 animals), and 6 weeks (7 animals). Block specimens were obtained using an inverted cone bur. Sections of 5μm were obtained and stained with Giemnsa.
Image acquiring was done with the use of a light microscope (21/3, Quimis) and camera (SDC-310) according to a previously published methodology16. Three randomly selected microscopic fields within each grafted area from all groups and animals were analysed.
Histomorphometric parameters were analysed using the UTHSCSA Image Tool 2.00. Areas (mm2) of grafted bone in relation to total area (two weeks only), areas of mature bone, immature bone, osteoyd (with osteoblastic profliferation), medullar regions (four and six weeks only) and granulation tissue were measured (Figure 2). A total of approximattely 2 mm2 were analysed in each field. Data were recorded in mm2 and percentage for each parameter.
Statistical analysis
Within each monitoring period (weeks), Friedman's nonparametric "t" test was done. In the case of significant difference among treatments, the Wilcoxon's nonparanetric test was done. Values of p<0.05 indicated statistical significance.
Results
Platelets counting analysis
Medium values, minimum and maximum values were obtained for all of the countings (Table 2).
The used method for the production of the highly concentrated PRP in this study allowed for a mean enrichment of 687% of platelets, wich means that the hPRP counting of platelets was approximately seven times higher than initial counting of platelets.
Morphological microscopic analysis
After two weeks, group 1 (APB) presented in the repair of bone defect a loose connective tissue mildly vascularized, with low cellularity, either of fusiform-like (similar to mature fibroblast) or of star-like aspect (similar to immature or young fibroblast, or even at an osteoblastic differentiation stage). It can also be observed, at the center of the defect, fragments of mature bone (probably from grafting) with osteoclastic activity. In the surrounding areas of the defect a neoformation of immature bone tissue was observed, with intense osteoblastic activity. The group 2 (APB + hPRP) presented a granulation tissue mildly vascularized in all cases, with intense cellularity, either of fusiform-like or star-like aspect, similar to young or immature fibroblast or in osteoblastic differentiation. Also, fragments of mature bone tissue (grafted origin) with osteoblastic and osteoclastic activity were noticed. In the surrounding areas, an immature bone neoformation with intense osteoblastic activity occurred. The group 3 (hPRP) presented a loose and dense connective tissue compatible to granulation tissue, with fusiform-like and osteoblastic cells. Also, intense and diffuse inflammatory process, especially eosinofilic, was observed within the defect. In the surrounding areas, intense osteoblastic activity with osteoyd tissue was seen. The group 4 (control) presented intense angiogenic area with dilated blood vessels. Within this area a trabecullar bone matrix in maturation stage was also found (Figure 2).
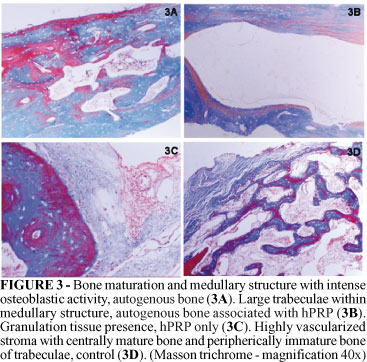
After four weeks the group 1 (APB) showed intense osteoblastic activity with hyalinized stroma similar to osteoyd. The group 2 (APB + hPRP) showed either loose or dense connective tissue, compatible with granulation tissue, similar to what was observed in two weeks, nevertheless more bone tissue was noticed peripherically. The group 3 (hPRP) showed similar histological aspects to two weeks, but without inflammatory process. Continue and immature bone trabeculae were observed within granulation tissue in the medullar region of defect. While in the group 4 (control) the aspect was also similar to two weeks, with mature bone trabeculae at central regions and immature bone peripherically, in a highly vascularized stroma (Figure 3).
After six weeks the group 1 (APB) demonstrated bone trabeculae in highly vascularized stroma. The group 2 (APB + hPRP) demonstrated bone tissue fragment similar to medullar aspect, likewise group 1 (APB), but with bone trabeculae of smaller widths. The group 3 (hPRP) demonstrated loose connective tissue highly vascularized with several fragments of bone tissue, with no trabeculae organization present yet. And finally, the group 4 (control) demonstrated a similar aspect to group 1, although with more sparse trabeculae layers (APB) (Figure 4).
Histomorphometric analysis
After two weeks, groups 1 (APB) and 2 (APB + hPRP) demonstrated means of 31.42% and 26.29%, respectively, of mature bone (MB) in relation to the total area of the defect. This mature bone found at this period demonstrated two degrees of bone; one considered grafted mature bone (GMB) and other neoformated mature bone (NMB). For these groups, the percentages of GMB were 23.87% and 19.25%, for groups 1 and 2 respectively; while the percentages of NMB were 7.60% and 7.03%, respectively. Also, the amounts of immature bone seen in both groups were 8.47% and 5.81%, for group 1 and 2. Comparing the groups 3 (hPRP) and 4 (control), group 4 presented a higher percentage of mature bone (3 = 7.10%; 4 = 9.40%); for immature bone the results showed 18.50% (group 3) and 20.93% (group 4) - not stastiscally significant.
After 4 weeks, the percentage of mature bone in group 2 (35.99%) was higher than in groups 3 (16.15%) and 4 (19.07%) - results statistically significant. In group 1, the presence of mature bone was 54.18%, significantly higher than all the other studied groups. Comparing the amounts of immature bone, group 4 showed the highest percentage (4 = 42.02%; 3 = 17.34%; 1 = 16.01%; 2 = 14.94%).
After 6 weeks, group 1 continued showing the highest percentage of mature bone when compared with all others (1 = 52.06%; 2 = 36.55%; 4 = 28.06%; 3 = 22.16%). Comparing the presence of immature bone, group 4 (control) showed the highest percentage (4 = 25.61%; 3 = 12.44%; 1 = 12.30%; 2 = 9.29%).
In relationship to the presence of granulation tissue, the groups with hPRP (groups 2 and 3) presented the highest percentages at four and six weeks; at four weeks, the amounts of granulation tissue were 3 = 31.00%, 2 = 25.91%, 1 = 7.70% and 4 = 6.83%; at six weeks the amounts were 3 = 20.28%, 2 = 8.46%, 1 = 2.41% and 4 = 1.94%.
Discussion
It is known that platelets are a source of several growth factors. The PDGF and the TGF-β seem to be the main growth factors in the PRP, once they are always mentioned1,2,3,7,9,12. Many doubts still remain regarding the effectiveness of the PRP and the growth factors on the mechanism of tissue repair.
In humans, many authors demonstrate positive results with the use of PRP in situations of tissue repair2,3,17,18-22, while others, however, do not observe benefits9,23.
Sanchez et al.24 affirm that there is a lack of scientific evidences to support the use of PRP in combination with graftings in reconstructive procedures, and Frymiller and Aghaloo8 establish doubts if PRP can be recommended for the use in humans.
In animals, several studies had been developed with application of PRP. Some of these studies also use rabbits5,25,26. Aghaloo et al.5 show a very similar study to this presented here, with differences in the preparation of the PRP, in the monitoring periods (two, four and six weeks here and one, two and four months in theirs) and in the results when in the current study the PRP seemed to be harmful to the repair, these authors practically demonstrate neutral action of the PRP.
Dogs also were used in studies with PRP. Kim et al.25 demonstrate positive results in the association of implants, dried frozen bovine bone and PRP. Suba et al.27 associate the beta-tricalcium phosphate to the PRP with positive results, even at initial phases of repair. Gerard et al.28 also demonstrate favorable results at initial periods of repair with the association of cortico-medular autogenous bone and PRP. Poor results are demonstrated by Choi et al.15, and for Jensen et al.29.
Sheep were used by Jakse et al.30 (autogenous bone and PRP) and by Sarkar et al.31 (collagen sponge and PRP) - in both the studies the results with PRP were not advantageous. Fürst et al.32, Wiltfang et al.18 and Klongnoi et al.33 use minipigs as experimental animal. Fürst et al.32 (bovine bone) and Klongnoi et al.33 (autogenous bone, bioactive glass) do not demonstrate positive results with PRP. However, Wiltfang et al.18 (autogenous bone) show positive results with the use of the PRP.
In goats, autogenous bone showed considerable improvements in the bone repair, with increase of the revascularization, reduction of the reabsortion and increase of the bone neoformation, mainly after six and 12 weeks; the beneficial effect of the PRP were also observed later on19. Although it is known that the growth factors are delivered during few days after the local application, according to these authors, their initial effect can be important once they can promote the revascularization and also tissue acceptance of the grafted material. In the current study, the opposite was observed; perhaps an initial problem with the revascularization or acceptance of the grafted material may be the key factor for the impaired results observed later.
In rats, Fontana et al.34 (titanium laminar implant), Pryor et al.35 (collagen sponge) and Plachokova et al.36 (beta-tricalcium phosphate, hidroxiapatite) did not find results that justify the use of PRP. However, Weibrich et al.14 (implants) while studying different levels of PRP concentration, found positive results with concentrations lower than six times, and observed that the use of highly concentrated PRP (6-11 times) inhibits the bone repair. The technique used for the production of the PRP in the current study was capable to produce mean platelets concentrations higher than six times the concentrations verified in the peripheral venous blood. Perhaps this high concentration has contributed for the poor results in this study.
Studies in vitro supply with interesting data regarding the PRP. Arpormaeklong et al.13 (rat bone marrow stromal cells, rhBMP2, PPP = platelet-poor plasma) demonstrated the PRP as estimulator of the cellular proliferation (dose-dependent), and inhibitor of the activity of alkaline phosphatase and calcium deposition. Choi et al.37 (alveolar bone cells, PPP, PC = platelets concentrate without plasma) observed that PRP in high concentrations suppress the viability and the cellular proliferation, however there is a stimulation effect in low concentrations (1-5%). Also observed cytotoxic answers with the PPP, and with PC an increase in the viability and the proliferation cellular of significant form. Such results raise the question about the plasma itself as responsible for the negative effects found; likewise the results observed in the current study, which could possibly be attributed to the amount of plasma in PRP.
Annunziata et al.38 report that PRP stimulates the growth of periodontal ligament human cells (dose-dependent) and the proliferation of gingival fibroblasts, also inhibits the growth of human keratinocytes and increases the activity of alkaline phosphatase. Biological effect of the PRP on the proliferation and differentiation of human osteoblast-like cells was already observed, increasing cell viability (dose-dependent) and suppressing the activity of alkaline phosphatase in the phase of cellular growth, although enhancing the enzyme action when cells reach confluence.
Graziani et al.6 studied the effect of different concentrations of PRP on human osteoblasts and fibroblasts. Maximum stimulation effects are found in the concentrations of 2.5x. Higher concentrations of PRP increase the amounts of TGF-b 1 and osteocalcin, and diminish the osteoprotegerin levels, stimulating osteoclastogenesis and osteoblastic differentiation; a nocive effect once during the initial phases of the repair it is essential that the proliferation occurs before the differentiation. These results also are in agreement with the non-favorable results found in the use of the highly concentrated PRP demonstrated in the current study.
Conclusion
The use of a highly concentrated PRP was considered prejudicial to the repair of non-critical defects in the calvaria of rabbits, either in the association of autogenous particled bone, when compared to autogenous particled bone alone, or in its isolated form, when compared to blood clot (control).
Received: August 18, 2009
Review: October 14, 2009
Accepted: November 18, 2009
Conflict of interest: none
Financial source: none
How to cite this article
Oliveira Filho MA, Nassif PAN, Malafaia O, Ribas Filho JM, Ribas CAPM, Camacho AC, Stieven Filho E, Giovanini AF. Effects of a highly concentrated platelet-rich plasma on the bone repair using non-critical defects in the calvaria of rabbits. Acta Cir Bras. [serial on the Internet] 2010 Jan-Feb;25(1). Available from URL: http://www.scielo.br/acb
- 1. Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55(11):1294-9.
- 2. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-46.
- 3. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14(4):529-35.
- 4. Petrungaro PS. Using platelet-rich plasma to accelerate soft tissue maturation in esthetic periodontal surgery. Compend Contin Educ Dent. 2001;22(9):729-32.
- 5. Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg. 2002;60(10):1176-81.
- 6. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17(2):212-9.
- 7. Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000;71(10):1654-61.
- 8. Freymiller EG, Aghaloo TL. Platelet-rich plasma: ready or not? J Oral Maxillofac Surg. 2004;62(4):484-8.
- 9. Shanaman R, Filstein MR, Danesh-Meyer MJ. Localized ridge augmentation using GBR and platelet-rich plasma: case reports. Int J Periodontics Restor Dent. 2001;21(4):345-55.
- 10. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-96.
- 11. Whitman DH, Berry RL. A technique for improving the handling of particulate cancellous bone and marrow grafts using platelet gel. J Oral Maxillofac Surg. 1998;56(10):1217-8.
- 12. Rosenberg ES, Torosian J. Sinus grafting using platelet-rich plasma-initial case presentation. Pract Periodontics Aesthet Dent. 2000;12(9):843-50.
- 13. Arpornmaeklong P, Kochel M, Depprich R, Kübler NR, Würzler KK. Influence of platelet-rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int J Oral Maxillofac Surg. 2004;33(1):60-70.
- 14. Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34(4):665-71.
- 15. Choi BH, Im CJ, Huh JY, Suh JJ, Lee SH. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg. 2004;33(1):56-9.
- 16. Zielak JC, Lopes DK, Giovanini AF, Baratto Filho F, Mathias AL. Histological evaluation of experimental bone grafting in vivo of lyophilized deproteinated bovine bone. Rev Sul-Bras Odontol. 2007;4:22-8.
- 17. Gehring S, Hoerauf H, Laqua H, Kirchner H, Klüter H. Preparation of autologous platelets for the ophthalmologic treatment of macular holes. Transfusion. 1999;39(2):144-8.
- 18. Wiltfang J, Schlegel KA, Schultze-Mosgau S, Nkenke E, Zimmermann R, Kessler P. Sinus floor augmentation with beta-tricalciumphosphate (beta-TCP): does platelet-rich plasma promote its osseous integration and degradation? Clin Oral Implants Res. 2003;14(2):213-8.
- 19. Oyama T, Nishimoto S, Tsugawa T, Shimizu F. Efficacy of platelet-rich plasma in alveolar bone grafting. J Oral Maxillofac Surg. 2004;62(5):555-8.
- 20. Okuda K, Tai H, Tanabe K, Suzuki H, Sato T, Kawase T, Saito Y, Wolff LF, Yoshiex H. Platelet-rich plasma combined with a porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans: a comparative controlled clinical study. J Periodontol. 2005;76(6):890-8.
- 21. Sammartino G, Tia M, Marenzi G, di Lauro AE, D'Agostino E, Claudio PP. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2005;63(6):766-70.
- 22. Mannai C. Early implant loading in severely resorbed maxilla using xenograft, autograft, and platelet-rich plasma in 97 patients. J Oral Maxillofac Surg. 2006;64(9):1420-6.
- 23. Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Kenney EB. Comparison of platelet-rich plasma, bovine porous bone mineral, and guided tissue regeneration versus platelet-rich plasma and bovine porous bone mineral in the treatment of intrabony defects: a reentry study. J Periodontol. 2002;73(2):198-205.
- 24. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
- 25. Kim ES, Park EJ, Choung PH. Platelet concentration and its effect on bone formation in calvarial defects: an experimental study in rabbits. J Prosthet Dent. 2001;86(4):428-33.
- 26. Butterfield KJ, Bennett J, Gronowicz G, Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005;63(3):370-6.
- 27. Suba Z, Takács D, Gyulai-Gaál S, Kovács K. Facilitation of beta-tricalcium phosphate-induced alveolar bone regeneration by platelet-rich plasma in beagle dogs: a histologic and histomorphometric study. Int J Oral Maxillofac Implants. 2004;19(6):832-8.
- 28. Gerard D, Carlson ER, Gotcher JE, Jacobs M. Effects of platelet-rich plasma on the healing of autologous bone grafted mandibular defects in dogs. J Oral Maxillofac Surg. 2006;64(3):443-51.
- 29. Jensen TB, Rahbek O, Overgaard S, Søballe K. Platelet rich plasma and fresh frozen bone allograft as enhancement of implant fixation. An experimental study in dogs. J Orthop Res. 2004;22(3):653-8.
- 30. Jakse N, Tangl S, Gilli R, Berghold A, Lorenzoni M, Eskici A, Haas R, Pertl C. Influence of PRP on autogenous sinus grafts. An experimental study on sheep. Clin Oral Implants Res. 2003;14(5):578-83.
- 31. Sarkar MR, Augat P, Shefelbine SJ, Schorlemmer S, Huber-Lang M, Claes L, Kinzl L, Ignatius A. Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials. 2006;27(9):1817-23.
- 32. Fürst G, Gruber R, Tangl S, Zechner W, Haas R, Mailath G, Sanroman F, Watzek G. Sinus grafting with autogenous platelet-rich plasma and bovine hydroxyapatite. A histomorphometric study in minipigs. Clin Oral Implants Res. 2003;14(4):500-8.
- 33. Klongnoi B, Rupprecht S, Kessler P, Thorwarth M, Wiltfang J, Schlegel KA. Influence of platelet-rich plasma on a bioglass and autogenous bone in sinus augmentation. An explorative study. Clin Oral Implants Res. 2006;17(3):312-20.
- 34. Fontana S, Olmedo DG, Linares JA, Guglielmotti MB, Crosa ME. Effect of platelet-rich plasma on the peri-implant bone response: an experimental study. Implant Dent. 2004;13(1):73-8.
- 35. Pryor ME, Polimeni G, Koo KT, Hartman MJ, Gross H, April M, Safadi FF, Wikesjö UM. Analysis of rat calvaria defects implanted with a platelet-rich plasma preparation: histologic and histometric observations. J Clin Periodontol. 2005;32(9):966-72.
- 36. Plachokova AS, van den Dolder J, Stoelinga PJ, Jansen JA. The bone regenerative effect of platelet-rich plasma in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res. 2006;17(3):305-11.
- 37. Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH. Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int J Oral Maxillofac Surg. 2005;34(4):420-4.
- 38. Annunziata M, Oliva A, Buonaiuto C, Di Feo A, Di Pasquale R, Passaro I, Guida L. In vitro cell-type specific biological response of human periodontally related cells to platelet-rich plasma. J Periodontol Res. 2005;40(6):489-95.
Publication Dates
-
Publication in this collection
04 Feb 2010 -
Date of issue
Feb 2010
History
-
Reviewed
14 Oct 2009 -
Received
18 Aug 2009 -
Accepted
18 Nov 2009