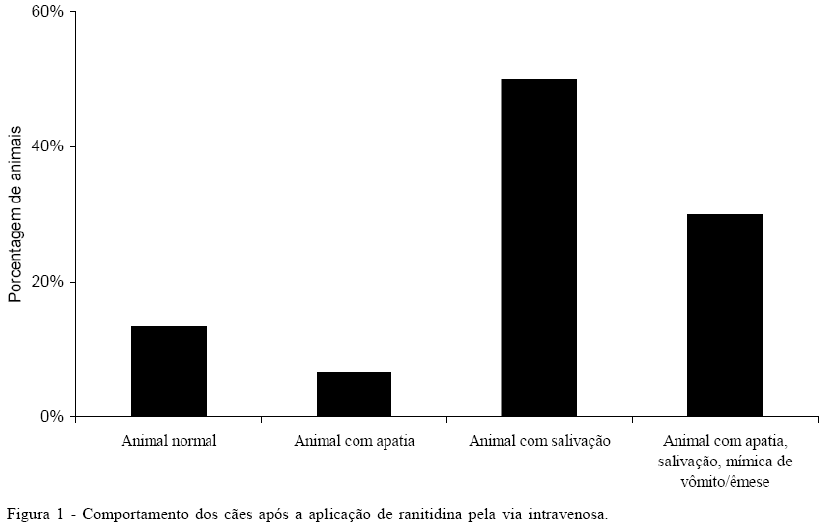
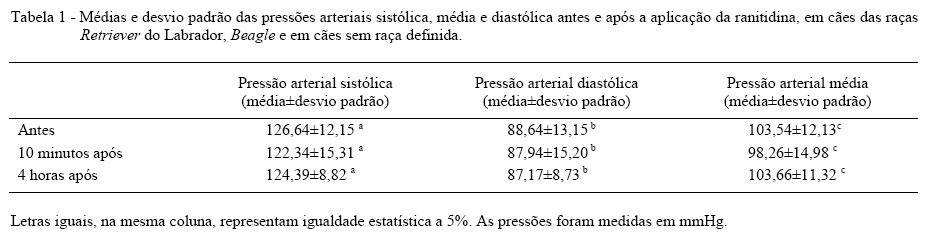
The purpose of this study was to verify if the ranitidine dosage of 2mg kg-1 by intravenous path causes emesis or hypotension in healthy dogs. They were selected 10 Labrador Retriever, 10 Beagles and 10 mongrel dogs, five animals of each sex. The animals were submitted to clinical examination and blood pressure evaluation before ranitidine administration and also 10 minutes and 4 hours after administration of it. After administration was observed that 13.3% of the animals presented normal; 6.7% of the dogs presented apathy; 50% of the animals presented salivation and 30% presented apathy, salivation, emesis mimic or emesis. There was no significative arterial blood pressure decrease after ranitidine administration. It was concluded that ranitidine useful in therapeutic dosage by intravenous path may provoke apathy, salivation, emesis mimic and emesis.
ranitidine; canines; undesired effects; emesis