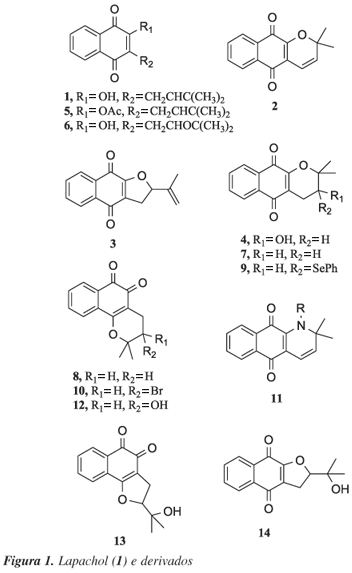
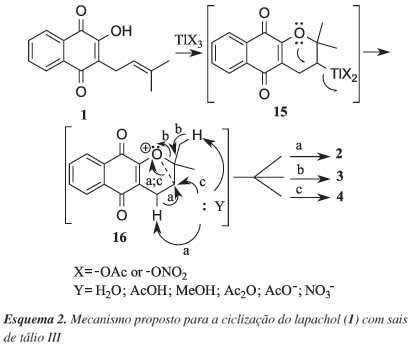
This work describes the cyclization of lapachol (1) induced by thallium triacetate (TTA) and thallium trinitrate (TTN) in several solvents using magnetic stirring and under microwave irradiation. alpha-Xyloidone (2) - dehydro-alpha-lapachone - was obtained as the main product in these reactions in 20 75% yield. However, rhinacanthin-A (4) was isolated as main product in a 40% yield, using TTA and acetic anhydride:water (1:1) as solvent, and dehydro-iso-alpha-lapachone (3) in 21% yield, using TTA and dichloromethane as solvent. The reaction time decreased drastically under microwave conditions, but the yields of these reactions were not the expected.
lapachol; thallium salts; cyclization