Abstracts
In an ex vivo agar plate assay, we monitored the appearance of an inhibitory halo against Vibrio cholerae from the feces of Wistar and Fischer rats aged 10 to 42 days. The frequency of Wistar rats showing halo increased from 0% (10 days) to a maximum of 80.0% (29 days) and then decreased to 53.3% (42 days). A similar pattern was obtained with Fischer rats but with a lower intensity (maximum frequency of 50.0% by day 36). In a separate experiment, when Wistar rats were fed a low-protein diet for 7 days, the inhibitory halo decreased drastically. Three apparently different colony morphologies were isolated from the dominant fecal microbiota: a facultative anaerobe (FAN) and two strict anaerobes (SAN). The ex vivo inhibitory test showed a halo around the feces of germfree mice monoassociated with the FAN bacterium or one of the SAN bacterium but not of the germfree ones. After oral challenge of all groups with V. cholerae, a permissive and a drastic barrier effects were observed in mice with FAN and SAN associated bacteria, respectively. The FAN and one SAN bacteria used in the in vivo challenges were identified as Escherichia coli and Streptococcus intermedius, respectively. The potent antagonism developed by the rat intestinal microbiota against V. cholerae seems to be due, in part, to diffusible compounds and this phenomenon depends apparently on age, strain and nutrition of the animals. These preliminary results also suggest that this effect was due to more than one bacterial component at any given moment.
Vibrio cholerae; Escherichia coli; Streptococcus intermedius; antagonism; rodents
O aparecimento de halo de inibição contra o Vibrio cholerae a partir das fezes de ratos Wistar e Fischer nas idades de 10 a 42 dias foi observado usando um teste ex vivo em placa. A frequência de ratos Wistar apresentando halo aumentou de 0% (10 dias) até um máximo de 80,0% (29 dias) antes de decair para 53,3% (42 dias). Um perfil similar foi obtido com os ratos Fischer mas com valores inferiores (frequência máxima de 50,0% no dia 36). Num experimento separado quando ratos Wistar foram alimentados com uma ração deficiente em proteína a freqüência de halo diminuiu drasticamente. Três morfologias de colonias aparentemente distintas foram isoladas da microbiota fecal dominante: uma bactéria anaeróbia facultativa (ANF) e duas anaeróbias estritas (ANS). O teste inibitório ex vivo mostrou halo ao redor das fezes de camundongos isentos de germes monoassociados com a bactéria ANF ou uma das bactérias ANS mas não para os animais isentos de germes. Após desafio oral de todos os grupos com o V. cholerae, barreiras permissiva e drástica foram observadas em camundongos associados com as bactérias ANF e ANS, respectivamente. As bactérias ANF e uma ANS usadas em monoassociação nos desafios in vivo foram identificadas como Escherichia coli e Streptococcus intermedius, respectivamente. O potente antagonismo demostrado pela microbiota intestinal de ratos contra V. cholerae parece devido, em parte, a compostos difusíveis e este fenômeno depende aparentemente da idade, de espécie e da nutrição do animal. Esses dados preliminares sugerem também que este antagonismo seja devido a mais de um componente bacteriano num instante dado.
Vibrio cholerae; Escherichia coli; Streptococcus intermedius; antagonism; ratos
ANTAGONISM AGAINST VIBRIO CHOLERAE BY BACTERIAL DIFFUSIBLE COMPOUND IN THE FECAL MICROBIOTA OF RODENTS
Simone Helena da Silva1, Enio Cardillo Vieira2, Jacques Robert Nicoli1*
1Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil; 2Departamento de Bioquímica-Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil
Approved: July 23, 1998
ABSTRACT
In an ex vivo agar plate assay, we monitored the appearance of an inhibitory halo against Vibrio cholerae from the feces of Wistar and Fischer rats aged 10 to 42 days. The frequency of Wistar rats showing halo increased from 0% (10 days) to a maximum of 80.0% (29 days) and then decreased to 53.3% (42 days). A similar pattern was obtained with Fischer rats but with a lower intensity (maximum frequency of 50.0% by day 36). In a separate experiment, when Wistar rats were fed a low-protein diet for 7 days, the inhibitory halo decreased drastically. Three apparently different colony morphologies were isolated from the dominant fecal microbiota: a facultative anaerobe (FAN) and two strict anaerobes (SAN). The ex vivo inhibitory test showed a halo around the feces of germfree mice monoassociated with the FAN bacterium or one of the SAN bacterium but not of the germfree ones. After oral challenge of all groups with V. cholerae, a permissive and a drastic barrier effects were observed in mice with FAN and SAN associated bacteria, respectively. The FAN and one SAN bacteria used in the in vivo challenges were identified as Escherichia coli and Streptococcus intermedius, respectively. The potent antagonism developed by the rat intestinal microbiota against V. cholerae seems to be due, in part, to diffusible compounds and this phenomenon depends apparently on age, strain and nutrition of the animals. These preliminary results also suggest that this effect was due to more than one bacterial component at any given moment.
Key words: Vibrio cholerae, Escherichia coli, Streptococcus intermedius, antagonism, rodents.
Salmonella typhimurium, Shigella flexneri Vibrio choleraeV. choleraeV. choleraeV. choleraeEscherichia coli, Proteus mirabilisEnterococcus faecalisV. choleraeThe antagonistic phenomenon is potent but also fragile, being perturbed by factors such as drug ingestion, feeding and stress (12). The possible mechanisms of this bacterial interaction include: competition for nutrients or for adhesion sites, stimulation of peristalsis and local immunity, and production of inhibitory substances (bacteriocins) or metabolites (fatty acids) (5).
This work describes the influence of age, sex, strain and low-protein nutrition on the production of a diffusible antagonistic substance against V. cholerae in the feces of rats. Isolation and partial identification of three bacteria producing these substances were also performed.
Animals and Diets.Germfree NIH mice (Taconic, Germantown, NY, USA) of both sexes were used as recipient animals for the screening of rat fecal bacteria responsible for the antagonistic compound production. Twenty-eight day old male Wistar rats fed a commercial rodent diet and whose fecal samples showed an inhibitory halo against V. cholerae were used as intestinal bacteria donors. The germfree animals were housed in flexible plastic isolators (Class Biologically Clean Ltd., Madison, WI, USA), and handled according to established procedures (9). The animals received sterilized water in square pack bottle (American Sterilizer Company, Erie, PA, USA) and a commercial autoclavable diet for rodent (Nuvital, Curitiba, PR, Brasil) ad libitum. Experiments with gnotobiotic mice were carried out in microisolators (UNO Roestvaststaal B.V., Zevenar, The Netherlands).
Experimental Design. To study the influence of age, strain and sex, we monitored the frequency of an inhibitory halo against V. cholerae from the feces of 100 Wistar or 100 Fischer rats of both sexes at the age of 10, 15, 22, 29, 36 and 42 days.
For the nutritional experiment, low-protein and control groups of 11 and 6 animals were used, respectively. The experimental diets were initiated for one week at the age of 22 days. After this time, both groups received the control diet for one more week. Inhibitory tests were performed at the age of 22, 29 and 36 days.
Detection of an Inhibitory Diffusible Substance by in vitro and ex vivo Tests. The in vitro assay for inhibitory diffusible substance was carried out by the double-layer method. The producing bacteria were spot inoculated on the surface of Brain Heart Infusion (BHI) agar or deMan, Rogosa and Sharpe (MRS) agar (Difco, Detroit, MI, USA) on a Petri dish. After incubation at 37
ooFor the ex vivo assay, freshly collected feces from rats (50 mg) or mice (10 mg) were placed on a Petri dish containing Thiosulfate-Citrate-Bile-Sucrose (TCBS) agar medium (Difco, Detroit, MI, USA) and incubated for 48 h at 4
o-1V. choleraeoV. choleraeScreening of Rat Fecal Bacteria Producing an Inhibitory Compound Against V. cholerae. The dominant fecal microbiota of rats was obtained by decimal dilution under incubation in an anaerobic chamber containing an atmosphere of 85% N
222-7oTMAssociation of Germfree Mice with Rat Fecal Bacteria. The three bacterial isolates were grown separately on BHI agar for 48 h at 37
oEx vivo V. cholerae8V. cholerae Counts. After oral challenge, freshly collected feces from gnotoxenic mice were submitted daily to decimal dilutions and counted on TCBS agar spread plates after 24 hr at 37
oStatistical Analysis. Inhibitory halo frequencies were compared by Fishers exact test. Values of halo diameters between the different ages of the same rat strain were analyzed by 1-way analysis of variance. When a significant difference was found among groups (P < 0.05), a multiple comparison test was used to determine differences between groups. Values of halo diameters between the strain of rats for the same age were compared by the two-tailed Student t test. Results were considered to be significantly different only when P < 0.05. Statistical analysis were performed using an EPISTAT software package (T.L. Gustafson, Round Rock, TX, USA).
in vitroin vivoin vitroin vivoEscherichia coliE. coliet al.Peptostreptococcusin vivoPeptostreptococcusin vivoFig. 1 shows that the frequency of Wistar rats of both sexes showing an inhibitory halo against V. cholerae from their feces increased significantly (P < 0.05) from 0% (10 days) to 80.0% (29 days) and then decreased to 53.3% (42 days). The values of halo diameter around these feces followed a similar pattern Fig. 2, increasing significantly (P < 0.05) from 3.19 ± 1.11 cm (15 days) to a maximum of 4.72 ± 1.24 cm (29 days) and then decreasing to 2.03 ± 0.58 cm (42 days). In Fischer rats, the frequencies of animals showing an inhibitory halo against V. cholerae were lower (Fig. 1) throughout the experiment, but the pattern was similar when compared with Wistar rats (maximum frequency of 50.0% by day 36). The halo diameter was also significantly smaller for Fischer rats (about 1 cm) when compared with their Wistar counterparts (P < 0.05), but relatively constant throughout the experiment (Fig. 2). Inhibitory halo frequency was similar in males and females throughout the experiment both in Wistar and Fischer rats (data not shown). Whereas the rat is naturally resistant to intestinal colonization by V. cholerae, the animals which did not show an inhibitory halo around their feces in the present study probably used a mechanism other than inhibitory diffusible compounds to eliminate the bacterial pathogen. The increase in inhibitory halo frequencies followed by a decrease observed in both Wistar and Fischer rats suggests a sequential colonization of the rat intestinal tract by different bacterial strains or a modification of the metabolic activity of the same bacterial strains as a function of age.
. Frequency of an inhibitory halo against Vibrio cholerae caused by a diffusible substance from feces of 10, 15, 22, 29, 36 and 42 day old Wistar or Fischer rats fed a commercial diet for rodents.
a,b,c Different letters indicate significant difference between the different ages for the same strain (P < 0.05).
1.2 Different numbers indicate significant differences between the two strains for the same age (P < 0.05).
. Diameter of the inhibitory halo against Vibrio cholerae caused by a diffusible substance from feces of 10, 15, 22, 29, 36 and 42 day old Wistar or Fischer rats fed a commercial diet for rodents.
a,b,c Different letters indicate significant differences between the different ages for the same strain (P < 0.05).
1.2 Different numbers indicate significant differences between the two strains for the same age (P < 0.05).
The gastrointestinal tract and its associated microbiota in human or animal beings constitute an open ecosystem with stable population and functional characteristics under normal environmental, physiological and nutritional conditions. Allogenic and autogenic factors can radically disturb this situation when the host is: (i) stressed in certain ways; (ii) is starved or exposed to other forms of acute malnutrition; (iii) is given certain drugs, especially antibacterial compounds (2). It is well known, for example, that the normal microbiota of antibiotic-treated or stressed animals is markedly altered, and the breakdown of regulatory mechanisms in the gastrointestinal ecosystem allows easier establishment of pathogens in the tract (4,13). The role of diet in modifications of the intestinal microbiota whithin the digestive ecosystem has been also the subject of numerous studies (10). In a separate experiment, when Wistar rats (22 days old and 63.64% frequency of inhibitory halo at the beginning of the experiment) were fed a low-protein diet for 7 days, the frequency of inhibitory haloes decreased significantly (P < 0.05) to 14% Fig. 3. After nutritional recovery with the control diet for one week, this frequency returned to 50.0%.
. Frequency of the inhibitory halo against Vibrio cholerae caused by a diffusible substance from feces of 22 day old Wistar rats fed a control diet or a low-protein diet for one week and then nutritionally recovered with the control diet for the next week.
a,b Different letters indicate significant differences between the two diets (P < 0.05).
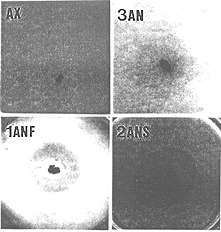
Using the above data, we selected 28 day old Wistar rats fed with a commercial diet for rodents and whose feces produced an inhibitory halo as fecal donor for the isolation of bacteria responsible for the antagonism against V. cholerae. From a 10-7 fecal dilution from this animal and after a 7 days incubation time at 37oC, we isolated 3 morphologically different colonies morphologies. The respiratory test showed that one of these bacteria was a facultative anaerobe (FAN) and the other two were strict anaerobes (SAN). These bacteria were associated with NIH germfree mice reared in microisolators. Five days later, the inhibitory test showed an antagonistic halo around the feces of the trixenic mice against the pathogenic bacterium (Fig. 4B). There was no halo from axenic animal feces (Fig 4A). Then, two germfree mice groups were respectively monoassociated with the FAN bacterium and diassociated with the two SAN bacteria in separate microisolators. Large inhibition zones were observed for the two groups (Figs. 4C and D) but at different times (after 5 and 15 days of association for monoxenic and dixenic animals, respectively). After oral challenge of monoassociated mice with V. cholerae, the bacterial pathogen was repressed (permissive barrier effect) to a population level of about 4.0 log CFU/g of feces in 3 days (Fig. 5). After the same oral challenge of diassociated mice, the bacterial pathogen was eliminated (drastic barrier effect) from the feces in about 2 weeks (Fig. 5). Then, germfree mice were associated for 5 days with one of the SAN bacteria when this microorganism reached fecal population levels of 9.6 log CFU/g. Smaller inhibition zones against V. cholerae were obtained from the feces of the monoassociated animals. Oral challenge of these mice with the bacterial pathogen resulted in its elimination from the mouse intestines in 5 days (Fig. 5). There was no inhibitory halo against the three pathogenic bacteria when an in vitro assay was carried out with them, independent of the medium used (BHI or MRS agar). This fact shows the limitation of the in vitro assay, because the production of inhibitory compounds against V. cholerae is apparently only possible in the host digestive ecosystem (as revealed by the ex vivo assay). Identification revealed Escherichia coli as the FAN strain. The two SAN bacteria were distinct Gram-positive cocci and the one used in the in vivo assay was identified as Streptococcus intermedius. The other SAN bacterium was an Extremely Oxygen Sensitive (EOS) strain. The presence in the rat gastrointestinal tract, at a given moment, of more than one antagonistic bacterial strain against V. cholerae showed that, as suggested by Freter (2), the complex interactive system regulating the indigenous microbiota and its colonization resistance property involves redundancy, an important characteristic for the protection of the ecosystem.
. Ex vivo inhibitory test against Vibrio cholerae in TCBS cholera medium using feces from germfree mice (AX), triassociated mice with one facultative anaerobic bacterium and two strict anaerobic bacteria (3AN), monoassociated mice with the facultative anaerobic bacterium (1ANF) or diassociated mice with the two strict anaerobic bacteria (2ANS) from the rat fecal microbiota.
The potent antagonism developed by the rat intestinal microbiota against V. cholerae seems to be due, in part, to diffusible compounds and this phenomenon depends apparently on the age, strain and nutrition of the animals. These preliminary results also suggest that the antagonistic effect of the rat intestinal microbiota against V. cholerae through diffusible substances, as observed in the ex vivo assay (but with different results from those obtained in the in vivo challenge, i.e., permissive and drastic barrier for the FAN and SAN bacteria, respectively), was exerted by more than one bacterial component at any given moment.
ACKNOWLEDGMENTS
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Financiadora de Estudos e Projetos (FINEP) and Pró-Reitoria de Pesquisa - UFMG (PRPq-UFMG). The authors thank Maria Gorete Barbosa Ribas for valuable technical assistance.
RESUMO
Antagonismo contra Vibrio cholerae por composto bacteriano na microbiota fecal de ratos
O aparecimento de halo de inibição contra o Vibrio cholerae a partir das fezes de ratos Wistar e Fischer nas idades de 10 a 42 dias foi observado usando um teste ex vivo em placa. A frequência de ratos Wistar apresentando halo aumentou de 0% (10 dias) até um máximo de 80,0% (29 dias) antes de decair para 53,3% (42 dias). Um perfil similar foi obtido com os ratos Fischer mas com valores inferiores (frequência máxima de 50,0% no dia 36). Num experimento separado quando ratos Wistar foram alimentados com uma ração deficiente em proteína a freqüência de halo diminuiu drasticamente. Três morfologias de colonias aparentemente distintas foram isoladas da microbiota fecal dominante: uma bactéria anaeróbia facultativa (ANF) e duas anaeróbias estritas (ANS). O teste inibitório ex vivo mostrou halo ao redor das fezes de camundongos isentos de germes monoassociados com a bactéria ANF ou uma das bactérias ANS mas não para os animais isentos de germes. Após desafio oral de todos os grupos com o V. cholerae, barreiras permissiva e drástica foram observadas em camundongos associados com as bactérias ANF e ANS, respectivamente. As bactérias ANF e uma ANS usadas em monoassociação nos desafios in vivo foram identificadas como Escherichia coli e Streptococcus intermedius, respectivamente. O potente antagonismo demostrado pela microbiota intestinal de ratos contra V. cholerae parece devido, em parte, a compostos difusíveis e este fenômeno depende aparentemente da idade, de espécie e da nutrição do animal. Esses dados preliminares sugerem também que este antagonismo seja devido a mais de um componente bacteriano num instante dado.
Palavras-chave: Vibrio cholerae, Escherichia coli, Streptococcus intermedius, antagonism, ratos.
REFERENCES
1. Berg, R.D. The indigenous gastrointestinal microflora. Trends Microbiol., 4:430-435, 1996.
2. Ducluzeau, R.; Raibaud, P. (1979) Ecologie microbienne du tube digestif. Masson, Paris, 1979, 96p.
3. Duval-Iflah, Y.; Raibaud, P.; Rousseau, M. Antagonism among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect. Immun., 34:957-969, 1981.
4. Freter, R. Experimental enteric Shigella and Vibrio infections in mice and guinea pigs. J. Exp. Med., 104:411-418, 1956.
5. Hentges, D.J. Role of the intestinal microflora in host defense against infection. In: Hentges, D.J. (ed). Human intestinal microflora in health and disease. Academic Press Inc., New York, 1983, p.311-331.
6. Horwitz, W. Official methods of analysis. Assoc. Offic. Analyt. Chem., Washington, 1980, 1018p.
7. Maier, B.R.; Hentges, D.J. Experimental Shigella infections in laboratory animals. I. Antagonism by human normal flora components in gnotobiotic mice. Infect. Immun., 6:168-173, 1972.
8. Miller, C.E.; Feeley, J.C. Competitive effects of intestinal microflora on Vibrio cholerae in gnotobiotic mice. Lab. Anim. Sci., 25:454-458, 1975.
9. Pleasants, J.R. Gnotobiotics. In: Melby Jr, E.C.; Altman, N.H. (eds). Handbook of laboratory animal science, CRC Press, Cleveland, 1974, p.119-173.
10. Raibaud, P.; Ducluzeau, R.; Muller, M.C.; Abrams, G.D. Diet and the equilibrium between bacteria and yeast implanted in gnotobiotic rats. Am. J. Clin. Nutr., 25:1457-1474, 1972.
11. Ramaré, F.; Nicoli, J.; Dabard, J.; Corring, T.; Ladiré, M.; Gueugneau, A.M.; Raibaud, P. Trypsin-dependent production of an antibacterial substance by a human Peptostreptococcus strain in gnotobiotic rats and in vitro. Appl. Environ. Microbiol., 59:2876-2883, 1993.
12. Savage, D. Factors influencing biocontrol of bacterial pathogens in the intestine. Food Technol., 41:82-87, 1987.
13. Tannock, G.W.; Savage, D.C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun., 9:591-598, 1976.
References
- 1 Berg, R.D. The indigenous gastrointestinal microflora. Trends Microbiol., 4:430-435, 1996.
- 2 Ducluzeau, R.; Raibaud, P. (1979) Ecologie microbienne du tube digestif Masson, Paris, 1979, 96p.
- 3 Duval-Iflah, Y.; Raibaud, P.; Rousseau, M. Antagonism among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect. Immun., 34:957-969, 1981.
- 4 Freter, R. Experimental enteric Shigella and Vibrio infections in mice and guinea pigs. J. Exp. Med., 104:411-418, 1956.
- 5 Hentges, D.J. Role of the intestinal microflora in host defense against infection. In: Hentges, D.J. (ed). Human intestinal microflora in health and disease Academic Press Inc., New York, 1983, p.311-331.
- 6 Horwitz, W. Official methods of analysis Assoc. Offic. Analyt. Chem., Washington, 1980, 1018p.
- 7 Maier, B.R.; Hentges, D.J. Experimental Shigella infections in laboratory animals. I. Antagonism by human normal flora components in gnotobiotic mice. Infect. Immun, 6:168-173, 1972.
- 8 Miller, C.E.; Feeley, J.C. Competitive effects of intestinal microflora on Vibrio cholerae in gnotobiotic mice. Lab. Anim. Sci., 25:454-458, 1975.
- 9 Pleasants, J.R. Gnotobiotics. In: Melby Jr, E.C.; Altman, N.H. (eds). Handbook of laboratory animal science, CRC Press, Cleveland, 1974, p.119-173.
- 10 Raibaud, P.; Ducluzeau, R.; Muller, M.C.; Abrams, G.D. Diet and the equilibrium between bacteria and yeast implanted in gnotobiotic rats. Am. J. Clin. Nutr., 25:1457-1474, 1972.
- 11 Ramaré, F.; Nicoli, J.; Dabard, J.; Corring, T.; Ladiré, M.; Gueugneau, A.M.; Raibaud, P. Trypsin-dependent production of an antibacterial substance by a human Peptostreptococcus strain in gnotobiotic rats and in vitro. Appl. Environ. Microbiol, 59:2876-2883, 1993.
- 12 Savage, D. Factors influencing biocontrol of bacterial pathogens in the intestine. Food Technol, 41:82-87, 1987.
- 13 Tannock, G.W.; Savage, D.C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun, 9:591-598, 1976.
Publication Dates
-
Publication in this collection
26 Feb 1999 -
Date of issue
Sept 1998
History
-
Accepted
23 July 1998 -
Reviewed
02 Mar 1998 -
Received
21 Oct 1997

 ANTAGONISM AGAINST VIBRIO CHOLERAE BY BACTERIAL DIFFUSIBLE COMPOUND IN THE FECAL MICROBIOTA OF RODENTS
ANTAGONISM AGAINST VIBRIO CHOLERAE BY BACTERIAL DIFFUSIBLE COMPOUND IN THE FECAL MICROBIOTA OF RODENTS