Abstracts
BACKGROUND AND OBJECTIVES: Human Bocavirus (HBoV) has been described since 2005 as an etiological agent of respiratory virus infections. From 2001 to 2008 we investigated the etiology of HBoV among adults and children in different groups at risk of presenting complications arising from acute respiratory infection, the investigation was carried out in a tertiary hospital health care system in Brazil. METHODS: HBoV DNA was assayed in 598 respiratory samples from community and hospitalized patients by PCR. RESULTS: Of the 598 tested samples, 2.44% (8/328) of children, including five children with heart disease, and 0.4% (1/270) of adult bone-marrow-transplant were HBoV positive. CONCLUSIONS: These data suggested lower HBoV frequency among different at-risk patients and highlights the need to better understand the real role of HBoV among acute respiratory symptomatic patients.
Human bocavirus; Risk groups; PCR
INTRODUÇÃO E OBJETIVOS: O bocavírus humano (HBoV) tem sido descrito desde 2005 como agente etiológico de infecções respiratórias virais. O presente estudo tem como objetivo investigar a etiologia da infecção respiratória pelo HBoV em pacientes adultos e crianças de diferentes grupos de risco para complicação de infecções respiratórias agudas desde 2001 até 2008 em um hospital terciário no Brasil. PACIENTES E MÉTODOS: O HBoV foi investigado, através de reação em cadeia da polimerase, em 598 amostras respiratórias coletadas de pacientes hospitalizados e não hospitalizados. RESULTADOS: Das 598 amostras testadas o HBoV foi detectado em 2,44% (8/328) das crianças, incluindo cinco crianças portadoras de cardiopatia congênita, e 0,4% (1/270) dos adultos em programa de transplante de células tronco hematopoiéticas. CONCLUSÃO: Os dados do presente estudo sugerem baixa freqüência de detecção do HBoV entre pacientes de risco, e destaca a necessidade de novos estudos para um melhor entendimento do verdadeiro papel desse agente em infecções respiratórias agudas em pacientes sintomáticos.
VIROLOGY
Frequency of human bocavirus respiratory infections among at-risk patients in São Paulo, Brazil
Frequência de bocavírus humano em infecções respiratórias entre pacientes de risco na cidade de São Paulo, Brasil
Elaine Regina Baptista CacciaI; Aripuana Sakurada Aranha WatanabeI; Emerson CarraroI,II; Elcio LealIII; Celso GranatoI; Nancy BelleiI
IClinical Virology Laboratory, Infectious Diseases Unit, Medicine Department, Sao Paulo Federal University, Sao Paulo, Brazil
IIMiddle-west State University, Guarapuava, Paraná, Brazil
IIIFederal University of Pará, Pará, Brazil
Correspondence Correspondence to: Aripuana Sakurada Aranha WATANABE, Universidade Federal de São Paulo/UNIFESP/EPM. Rua Pedro de Toledo 781, 15° andar, Vila Clementino. 04039-032 São Paulo, SP, Brasil. Tel.: 55.11.5081-5394. Fax: 55.11.5081-5394. E-mail: aripuana77@hotmail.com
SUMMARY
BACKGROUND AND OBJECTIVES: Human Bocavirus (HBoV) has been described since 2005 as an etiological agent of respiratory virus infections. From 2001 to 2008 we investigated the etiology of HBoV among adults and children in different groups at risk of presenting complications arising from acute respiratory infection, the investigation was carried out in a tertiary hospital health care system in Brazil.
METHODS: HBoV DNA was assayed in 598 respiratory samples from community and hospitalized patients by PCR.
RESULTS: Of the 598 tested samples, 2.44% (8/328) of children, including five children with heart disease, and 0.4% (1/270) of adult bone-marrow-transplant were HBoV positive.
CONCLUSIONS: These data suggested lower HBoV frequency among different at-risk patients and highlights the need to better understand the real role of HBoV among acute respiratory symptomatic patients.
Keywords: Human bocavirus; Risk groups; PCR.
RESUMO
INTRODUÇÃO E OBJETIVOS: O bocavírus humano (HBoV) tem sido descrito desde 2005 como agente etiológico de infecções respiratórias virais. O presente estudo tem como objetivo investigar a etiologia da infecção respiratória pelo HBoV em pacientes adultos e crianças de diferentes grupos de risco para complicação de infecções respiratórias agudas desde 2001 até 2008 em um hospital terciário no Brasil.
PACIENTES E MÉTODOS: O HBoV foi investigado, através de reação em cadeia da polimerase, em 598 amostras respiratórias coletadas de pacientes hospitalizados e não hospitalizados.
RESULTADOS: Das 598 amostras testadas o HBoV foi detectado em 2,44% (8/328) das crianças, incluindo cinco crianças portadoras de cardiopatia congênita, e 0,4% (1/270) dos adultos em programa de transplante de células tronco hematopoiéticas.
CONCLUSÃO: Os dados do presente estudo sugerem baixa freqüência de detecção do HBoV entre pacientes de risco, e destaca a necessidade de novos estudos para um melhor entendimento do verdadeiro papel desse agente em infecções respiratórias agudas em pacientes sintomáticos.
INTRODUCTION
Human bocavirus (HBoV) infections have been reported in children with upper and subsequent lower respiratory tract infections, with rates ranging from 1.4% to 19%2. There is a lack of information about the role of this pathogen in the etiology of acute respiratory infections (ARI) among patients in different risk groups. Studies among adult lung-transplant recipients or other immunocompromised patients detected a rate of 3.1% HBoV infection9. SCHENK et al. (2007)19 reported disseminated HBoV infection in a pediatric patient weeks after hematopoietic stem-cell transplantation. The aim of the study was to investigate the etiology of HBoV among adults and children in different groups at risk of presenting acute respiratory infections from 2001 to 2008.
PATIENTS AND METHODS
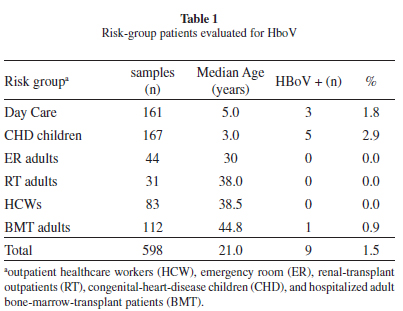
Population: Five hundred and ninety-eight samples collected from five different risk groups between 2001 and 2008 were assayed for DNA HBoV. Patients with a clinical diagnosis of Acute Respiratory Infection (ARI), up to seven days after onset of symptoms, of possible viral etiology (as determined by a physician), were considered eligible. Subjects were enrolled on a daily basis whenever a physician contacted the study team. The included patients were interviewed by the research staff, and information about demographic data, history of symptoms, clinical presentation, comorbidities, and influenza vaccination status were collected. Overall, 328 children (mean, median and range: 2.4 years old, one year old, 22 days-11 years old) and 270 adults (mean, median and range: 40.9, 42, 15-83 years old), with a median age of 21 years (ranging from 22 days to 83 years), were evaluated by a physician who collected samples (nasal wash for adults and nasopharyngeal aspirate for children) and sent them to the Virology Laboratory of the Sao Paulo Federal University. This study was approved by the Ethics Committee of Sao Paulo Federal University, and written consent was obtained from all patients or from those responsible for the individual patient.
Yearly Recruitment centers: From 2001 to 2003, 83 outpatient healthcare workers (HCW), 44 adult patients from the emergency room (ER) and 31 renal-transplant outpatients (RT) were evaluated; 64 ambulatory pediatric patients with congenital heart disease (CHD) were sampled in 2005, and in 2008 we received samples from 161 children from a day-care center, 103 CHD children, and 112 hospitalized adult bone-marrow-transplant patients (BMT). All demographic and clinical data were recorded.
All 598 samples were prospectively investigated for antigen detection of influenza A and B, parainfluenza (types 1-3), adenovirus, and RSV by Direct Fluorescence Assay (DFA). Negative samples of the adults recruited first (2001-2003) were also tested for human metapneumovirus (hMPV), human rhinovirus (HRV), enterovirus (HEV), and coronavirus (HCoV) 229E and OC43, as previously described3, and those without another virus were tested for HBoV.
DNA HBoV detection and nucleotide sequencing: Viral DNA was extracted using the QIAmp Blood DNA Mini kit (Qiagen, USA) according to the manufacturer's instructions. HBoV DNA detection was performed by PCR for the NP-1 gene amplification as described elsewhere, with minor modifications12. As a positive control, we used a HBoV PCR product cloned into the plasmid pCRII-TOPOXL (Invitrogen, USA). The assay was standardized for detection at 40 copies of plasmid per reaction. All HBoV-positive samples were confirmed by sequencing with the same PCR primers. Sequencing reactions were performed using the Big Dye Terminator kit (Applied Biosystems, USA) and the ABI Prism 3130XL DNA sequencer. The sequences were aligned using the ClustalX program12. Phylogenetic relationships for the NP-1 sequences were assessed using maximum likelihood (ML) methods. Trees were replicated 100 times to provide bootstrap support for clades. The analysis was performed using Topali v2.5 software16. Eleven published HBoV sequences within the NP-1 region were obtained from the GenBank database (NCBI).
RESULTS
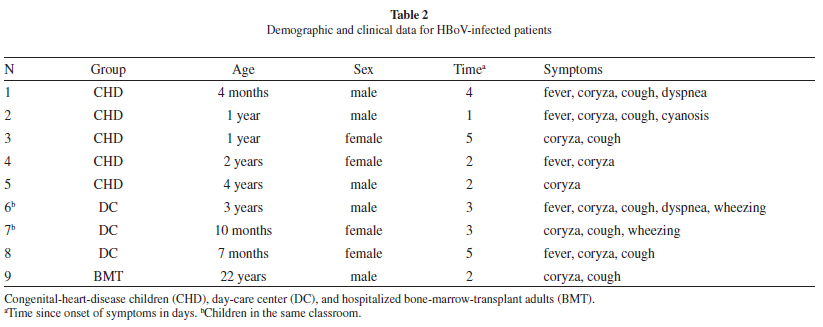
We obtained nine (1.5%) HBoV-DNA positives from 598 respiratory samples. Table 1 shows the study population characteristics and HBoV-infected patients. Overall, 2.44% of the children (8/328) and 0.40% of the adults (1/270) were HBoV-DNA-positive: no positive case was found among emergency-room patients. Positive patients (7/9; 77%) had samples collected within five days of symptom onset (a median of three days). The clinical data presented by HBoV-infected patients are shown in Table 2.
Distribution of positive samples in the period of study was as follows: 66.7% (6/9) were detected in autumn, 11.1% (1/9) in summer, 11.1% (1/9) in winter, and 11.1% (1/9) in spring.
Among the positive cases, 77% (7/9) presented coryza and cough, and 55% (5/9) presented fever. Among the positive pediatric cases, 25% (2/8) presented dyspnea and wheezing, and 12.5% (1/8) presented cyanosis. Overall, the patients with HBoV infection presented mild outcomes. None of the HBoV-infected patients required hospitalization or other supported therapy. Patients 6 and 7 (Table 2) were in close contact in the same classroom and had a one-day interval between symptom onset. All positive samples were sequenced, and six were assessed through phylogenetic analysis. Five out of six samples clustered with HBoV 1. One sample did not cluster with any of the reference sequences, probably due to a low sequence quality. Four of the obtained sequences were deposited in GenBank (accession numbers GQ244405.1, GQ244406.1, GQ244407.1, and GQ244408.1).
DISCUSSION
Overall, the detection found in our study (1.5%) was similar to those published elsewhere - 1.4% to 19%7. The majority of studies have been performed among hospitalized patients and nosocomial infection was also reported6.
HBoV infection occurred differentially among distinct risk groups of patients with acute respiratory symptoms; infections occurred more frequently among outpatient children than in adults, mainly among children with congenital heart disease. However, none of the CHD patients had a worse outcome or were hospitalized. One of few studies in heart-disease patients reported an 18.8% occurrence of HBoV4, but these cases were hospitalized children.
Previous studies found no HBoV infection among symptomatic adults1, and others reported frequencies of less than 1% among those and/or the elderly14,15. Although GUIDO et al. (2011) described an HBoV frequency of 18.9% among immunocompetent adults10. Other authors have suggested that HBoV infection may be a cause of hospitalization in the immunocompromised adult population4,14. We found only one adult bone-marrow-transplant case of HBoV infection, who presented mild disease even though coinfected with metapneumovirus.
In the present study HBoV were found circulating all year round, with an increased occurrence in late autumn. GAGLIARDI et al. (2009) in a study performed in the same geographical region (Brazilian southwest), found a similar autumn seasonality pattern8. Other viruses circulating during the same period in Brazil, such as HRSV17 and HRV20, possibly lead physicians to misdiagnose some illnesses as HboV, it may also account for some coinfections.
Sequences analysis confirmed that a single strain of HBoV circulated during the study period and that it was similar to strains described worldwide11,13. There was no difference in the strains circulating among children and adults, including the two HBoV-infected children in the same classroom.
The study presented was carried out as a hospital-based surveillance but the included patients were not hospitalized, except for the BMT population. The inclusion of inpatients (BMT) and outpatients (HCWs, children from a day-care center and CHD children) may have reduced the selection bias.
The inclusion of a control group of asymptomatic patients was not accomplished in the present investigation due to ethical questions related to healthy children sampling. Indeed, the retrospective design of the study would not allow the inclusion of a new patient group.
One of the study's limitations was that viral loads were not available since we tested samples by qualitative PCR and the possible roles of these viruses in coinfections were not assessed. PROENÇA-MODENA et al. (2011) used a quantitative PCR assay targeting the HBoV mRNA and described the probable HBoV pathogenicity in coinfections if higher mRNA loads occurred, probably indicating an active replication18. Another limitation was the long study period but we assumed that the collection of appropriate samples and appropriate storage could minimize possible bias.
Recently, ALLANDER et al. (2007) discussed a model in which primary HBoV infection occurs in early life and is a systemic infection associated with respiratory symptoms2. The infection seems to be frequently followed by asymptomatic low level virus shedding in the respiratory tract. Following this point of view we could speculate that this biological dynamic would explain the low frequency of single HBoV infection we obtained and the absence of infection among immunocompetent adult patients.
CONCLUSION
In conclusion, our results showed that HBoV infection among risk groups was lower, including highly immunosuppressed patients, and this highlights the need to better understand the real role of the HBoV among acute respiratory symptomatic patients.
ACKNOWLEDGMENT
We thank Dr. Eurico Arruda at Sao Paulo State University for the donation of the positive control.
FUNDING
This study was supported by the Sao Paulo State Research Foundation (FAPESP 2008/50352-2) and by the Brazilian National Research Council (CNPq).
CONFLICT OF INTEREST
None of the authors has declared a conflict of interest.
ETHICAL APPROVAL
This study was approved by the Ethics Committee of Sao Paulo Federal University (CEP 0471/2008), and written consent was obtained from all patients or those responsible for the individual patient.
Received: 15 March 2012
Accepted: 15 June 2012
This work was performed in Federal University of Sao Paulo. Rua Pedro de Toledo 781, 15°, Vila Clementino, 04339-032 S. Paulo, SP, Brazil. Tel.: 55.11.5081-5394.
- 1. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102:12891-6.
- 2. Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904-10.
- 3. Bellei N, Carraro E, Perosa A, Watanabe A, Arruda E, Granato C. Acute respiratory infection and influenza-like illness viral etiologies in Brazilian adults. J Med Virol. 2008;80:1824-7.
- 4. Chow BD, Huang YT, Esper FP. Evidence of human bocavirus circulating in children and adults, Cleveland, Ohio. J Clin Virol. 2008;43:302-6.
- 5. Dina J, Vabret A, Gouarin S, Petitjean J, Lecoq J, Brouard J, et al. Detection of human bocavirus in hospitalised children. J Paediatr Child Health. 2009;45:149-53.
- 6. Durigon GS, Oliveira DB, Vollet SB, Storni JG, Felício MC, Finelli C, et al. Hospital-acquired human bocavirus in infants. J Hosp Infect. 2010;76:171-3.
- 7. Esposito S, Bosis S, Niesters HG, Tremolati E, Sabatini C, Porta A, et al. Impact of human bocavirus on children and their families. J Clin Microbiol. 2008;46:1337-42.
- 8. Gagliardi TB, Iwamoto MA, Paula FE, Proença-Modena JL, Saranzo AM, Criado MF, et al. Human bocavirus respiratory infections in children. Epidemiol Infect. 2009;137:1032-6.
- 9. Garbino J, Soccal PM, Aubert JD, Rochat T, Meylan P, Thomas Y, et al. Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax. 2009;64:399-404.
- 10. Guido M, Quattrocchi M, Campa A, Zizza A, Grima P, Romano A, et al. Human metapneumovirus and human bocavirus associated with respiratory infection in Apulian population. Virology. 2011;417:64-70.
- 11. Hishinuma-Igarashi I, Mizuta K, SaitoY, Ohuchi Y, Noda M, Akiyama M, et al. Phylogenetic analysis of human bocavirus (HBoV) detected from children with acute respiratory infection in Japan. J Infect. 2009;58:311-3.
- 12. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947-8.
- 13. Lin JH, Chiu SC, Lin YC, Chen HL, Lin KH, Shan KH, et al. Clinical and genetic analysis of human bocavirus in children with lower respiratory tract infection in Taiwan. J Clin Virol. 2009;44:219-24.
- 14. Longtin J, Bastien M, Gilca R, Leblanc E, de Serres G, Bergeron MG, et al. Human bocavirus infections in hospitalized children and adults. Emerg Infect Dis. 2008;14:217-21.
- 15. Maggi F, Andreoli E, Pifferi M, Meschi S, Rocchi J, Bendinelli M. Human bocavirus in Italian patients with respiratory diseases. J Clin Virol. 2007;38:321-5.
- 16. Milne I, Wright F, Rowe G, Marshall DF, Husmeier D, McGuire G. TOPALi: software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics. 2004;20:1806-7.
- 17. Pecchini R, Berezin EN, Felício MC, Passos SD, Souza MC, Lima LR, et al. Incidence and clinical characteristics of the infection by the respiratory syncytial virus in children admitted in Santa Casa de São Paulo Hospital. Braz J Infect Dis. 2008;12:476-9.
- 18. Proença-Modena JL, Gagliardi TB, Escremim de Paula F, Iwamoto MA, Criado MF, Camara AA, et al. Detection of human bocavirus mRNA in respiratory secretions correlates with high viral load and concurrent diarrhea. PLoS One. 2011;6(6):e21083.
- 19. Schenk T, Strahm B, Kontny U, Hufnagel M, Neumann-Halfelin D, Falcone V. Disseminated bocavirus infection after stem cell transplant. Emerg Infect Dis. 2007;13:1425.
- 20. Watanabe A, Carraro E, Kamikawa J, Leal E, Granato C, Bellei N. Rhinovirus species and their clinical presentation among different risk groups of non-hospitalized patients. J Med Virol. 2010;82:2110-5.
Publication Dates
-
Publication in this collection
12 Nov 2012 -
Date of issue
Dec 2012
History
-
Received
15 Mar 2012 -
Accepted
15 June 2012