Abstracts
The multivariate chemical analysis of essential oils of Eugenia uniflora leaves with different fruit colours indicated the presence of three oil clusters regarding sample biotypes. The first cluster included yellowish, dark red and purple fruits with high percentages of germacrene B (11.1-30.7%), germacrone (9.8-54%) and atractylone (0-19.9%). In cluster II, with bright red fruit samples, the major constituents were curzerene (42.0-43.2%), germacrene D (8.7-9.0%) and germacrene A (5.9-8.9%), whereas cluster III included red-orange fruit samples containing high contents of selina-1,3,7(11)-trien-8-one (40.3-55.4%) and selina-1,3,7(11)-trien-8-one epoxide (12.7-24.4%). The clustered oils were investigated against the systemic fungus Paracoccidioides brasiliensis via the broth macrodilution method. The oil from cluster II revealed the most significant result. The yeast form of P. brasiliensis was completely inhibited at a concentration of 62.5 µg mL-1.
Eugenia uniflora; essential oil; chemical variability; antifungal activity; Paracoccidioides brasiliensis
A análise multivariada da composição química dos óleos essenciais das folhas de Eugenia uniflora com diferentes cores de fruto indicou a presença de três grupos de óleos em relação ao biótipo do fruto das amostras. O primeiro grupo incluiu amostras de frutos amarelos, vermelhos escuros e roxos contendo altas percentagens de germacreno B (11,1-30,7%), germacrona (9,8-54%) e atractilona (0-19,9%). No grupo II, com amostras de frutos vermelhos claro, os constituintes majoritários foram o curzereno (42,0-43,2%), germacreno D (8,7-9,0%) e germacreno A (5,9-8,9%), enquanto que o grupo III incluiu amostras com frutos vermelho-alaranjado, caracterizadas por um alto conteúdo de selina-1,3,7(11)-trien-8-ona (40,3-55,4%) e epóxido de selina-1,3,7(11)-trien-8-ona (12,7-24,4%). Os óleos essenciais foram investigados frente ao fungo Paracoccidioides brasiliensis pela técnica de macrodiluição em caldo. O resultado mais significativo foi obtido com o óleo do grupo II, com a forma leveduriforme de P. brasiliensis sendo inibida completamente na concentração de 62,5 µg mL-1.
ARTICLE
Influence of fruit biotypes on the chemical composition and antifungal activity of the essential oils of eugenia uniflora leaves
Deomar P. CostaI; Elenilson G. Alves FilhoI; Lorena M. A. SilvaI; Suzana C. SantosI; Xisto S. PassosII,IV; Maria do Rosário R. SilvaII; José C. SeraphinIII; Pedro H. Ferri* * e-mail: pedro@quimica.ufg.br ,I
IInstituto de Química
IIInstituto de Patologia Tropical e Saúde Pública
IIIInstituto de Matemática e Estatística, Universidade Federal de Goiás, CP 131, 74001-970 Goiânia-GO, Brazil
IVInstituto de Ciências da Saúde, Universidade Paulista, 74845-090 Goiânia-GO, Brazil
ABSTRACT
The multivariate chemical analysis of essential oils of Eugenia uniflora leaves with different fruit colours indicated the presence of three oil clusters regarding sample biotypes. The first cluster included yellowish, dark red and purple fruits with high percentages of germacrene B (11.1-30.7%), germacrone (9.8-54%) and atractylone (0-19.9%). In cluster II, with bright red fruit samples, the major constituents were curzerene (42.0-43.2%), germacrene D (8.7-9.0%) and germacrene A (5.9-8.9%), whereas cluster III included red-orange fruit samples containing high contents of selina-1,3,7(11)-trien-8-one (40.3-55.4%) and selina-1,3,7(11)-trien-8-one epoxide (12.7-24.4%). The clustered oils were investigated against the systemic fungus Paracoccidioides brasiliensis via the broth macrodilution method. The oil from cluster II revealed the most significant result. The yeast form of P. brasiliensis was completely inhibited at a concentration of 62.5 µg mL-1.
Keywords:Eugenia uniflora, essential oil, chemical variability, antifungal activity, Paracoccidioides brasiliensis
RESUMO
A análise multivariada da composição química dos óleos essenciais das folhas de Eugenia uniflora com diferentes cores de fruto indicou a presença de três grupos de óleos em relação ao biótipo do fruto das amostras. O primeiro grupo incluiu amostras de frutos amarelos, vermelhos escuros e roxos contendo altas percentagens de germacreno B (11,1-30,7%), germacrona (9,8-54%) e atractilona (0-19,9%). No grupo II, com amostras de frutos vermelhos claro, os constituintes majoritários foram o curzereno (42,0-43,2%), germacreno D (8,7-9,0%) e germacreno A (5,9-8,9%), enquanto que o grupo III incluiu amostras com frutos vermelho-alaranjado, caracterizadas por um alto conteúdo de selina-1,3,7(11)-trien-8-ona (40,3-55,4%) e epóxido de selina-1,3,7(11)-trien-8-ona (12,7-24,4%). Os óleos essenciais foram investigados frente ao fungo Paracoccidioides brasiliensis pela técnica de macrodiluição em caldo. O resultado mais significativo foi obtido com o óleo do grupo II, com a forma leveduriforme de P. brasiliensis sendo inibida completamente na concentração de 62,5 µg mL-1.
Introduction
Fungal infections are common in tropical countries and may have a major impact on public health. Whereas the dermatophyte fungi group is of common occurrence,1 the incidence of systemic infections due to Paracoccidioides brasiliensis (Splendore) Almeida has increased in the last two decades.2P. brasiliensis is an etiological agent of paracoccidioidomycosis, the most prevalent human systemic mycosis in Latin America, where up to 10 million individuals are estimated to be infected.3 This thermally dimorphic fungus grows as a filamentous saprobe (mycelium) in the soil at 25 °C and as a multicelular parasitic form (yeast) in the host. The shift from the mycelial to the yeast form (36 °C) is crucial for the infection of the human host. The heat shock response of P. brasiliensis to an abrupt increase in environmental temperature during infection results in the expression of the heat shock protein family of 60 (HSP60) and 70 kDa (HSP70).4 HSPs are regarded as suitable targets for antimicrobial drug development.5
A previous phytochemical study of Eugenia uniflora L. (Myrtaceae) leaves has identified that macrocyclic ellagitannin dimmer oenothein B is responsible for the inhibition of HSP60 and (1,3)-β-glucan synthase (Pbfks1) transcript of P. brasiliensis.6 In addition, the yeast phase was inhibited by non-polar fractions of the leaf ethanolic extract constituted by terpenoids, mainly oxygenated sesquiterpenes.7 Curzerene (isofuranegermacrene), germacrene B and atractylone were the major constituents, all previously identified in the essential oils of E. uniflora and probably responsible for the antifungal activity.8-12E. uniflora leaves have been used in folk medicine for the treatment of diarrhoea,13 hyperglycemia, hyperlipidemia and hypertension.14 Furthermore, they have been used as an antimalarial and spasmolytic agent as well as enzyme inhibitors.15-17 Antimicrobial activities have also been reported for leaf essential oils against bacterial and dermatophyte isolates.18
The aim of this study was to investigate the chemical constituents of the essential oils from E. uniflora leaves with different fruit colour biotypes, in order to provide evidence for the existence of varieties of this species and to investigate whether these biotypes/chemotypes express a similar antifungal activity against P. brasiliensis. Essential oils from mature leaves of representative populations with yellowish, red (bright red, red-orange and dark red) and purple fruit colours were analyzed by GC-MS. To study chemical variability, chemical constituents were submitted to multivariate analysis to detect the pattern distribution of samples and to identify which constituents are able to distinguish between these groups of individuals. Representative clustered oils were submitted to an antifungal susceptibility assay with the yeast form of P. brasiliensis via the standard broth macrodilution method, according to the National Committee for Clinical Laboratory Standard.
Results and Discussion
Fruit colour variations are well known in E. uniflora, although varieties according to fruit colour biotypes are not easily definable.19 Previous studies have mainly focused on the essential oils of leaves and only a few have investigated oils extracted from fruits.8-12,20-22 The description of the colour of sampled fruits is frequently excluded from these studies.11 According to a recent study, the essential oil of two fruit colour biotypes from both leaves and fruits of E. brasiliensis Lam. may indicate the presence of the two chemotypes of this species.23
In this study, E. uniflora leaf essential oils were obtained from species with three main fruit colour biotypes: yellowish, red (bright red, red-orange and dark red) and purple.24 A total of 36 compounds were identified, accounting for 91.3-94.6% of volatile constituents (Table 1). The essential oils predominantly contained a sesquiterpene hydrocarbon composition, even though the oxygenated sesquiterpene content for some samples was over 75.5%. Essential oils from E. uniflora have showed that, although the main constituent may vary, sesquiterpenes are usually the dominant class.8-12,22 Nevertheless, leaf oils from plants growing in Argentina whose main constituents were the monoterpenes pulegone, carvone and limonene and the sesquiterpene nerolidol have been described.20
Results obtained from PCA (18 samples × 12 variables = 216 data) and nearest neighbour complete linkage cluster analysis using Wards technique revealed high chemical variability between leaf essential oil biotypes. Figure 1 shows the relative position of the individuals to an axial system originated in the PCA. The first PC accounts for 53.1% of total variance and discriminates well above the 99% confidence level the oxygenated sesquiterpenes of red-orange fruit colour samples from other biotypes. The second PC discriminates (p < 0.0001) bright red fruit samples by their high sesquiterpene hydrocarbon content.
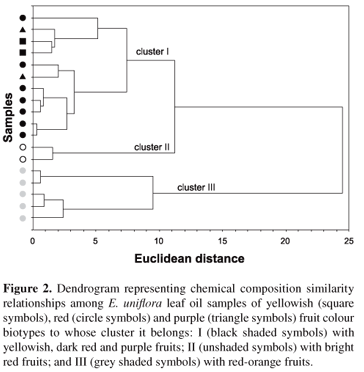
Therefore, three types of leaf essential oils were found: cluster I (samples with yellowish, dark red and purple fruit colours) was characterised (p < 0.015) by a high percentage of germacrene B (21.6 ± 7.2%), atractylone (11.7 ± 5.3%), germacrone (17.3 ± 13.2%) and b-caryophyllene (8.7 ± 3.7%); cluster II (samples with bright red fruit colour) had curzerene (42.6 ± 0.9%), germacrene D (8.8 ± 0.1%), germacrene A (7.4 ± 1.5%) and b-elemene (4.4 ± 0.1%) as principal constituents (p < 0.003); and cluster III (samples with red-orange fruit biotypes) contained high amounts (p < 0.029) of selina-1,3,7(11)-trien-8-one (48.2 ± 5.7%), selina-1,3,7(11)-trien-8-one epoxide (19.3 ± 4.3%), spathulenol (4.1 ± 5.0%) and d-selinene (3.3 ± 2.9%). Single Ion Monitoring (SIM) GC-MS analysis indicated that samples from clusters I and II did not show selina-1,3,7(11)-trien-8-one or their epoxide derivative, whereas samples from cluster III detected curzerene, germacrene B and atractylone. The latter was also verified in cluster II samples by the CG-MS-SIM technique. All similarities between sampled oil constituents are shown in the dendrogram of Figure 2.
In order to assimilate the overall trend in volatile leaf oils and to reduce the uncontrolled factors affecting quantitative variations, the constituent data were also coded as independent single characters (either present or absent), as recommended by Sneath and Sokal.25 Multiple Correspondence Analyses revealed a similar chemical distinction between the biotypes sampled.26 Clusters I and II were clustered and cluster III was split based on up and down samples in relation to the second PC origin, as shown in Figure 1 (data not shown).
The canonical discriminant analysis (CDA) confirmed the clustering as a priori groupings. Furthermore, an axial system that originated in the CDA discriminated well above the 99% confidence level the three types of essential oils based only on the contents of curzerene and selina-1,3,7(11)-trien-8-one as predictor variables (Table 2). The first discriminant function (F1) accounts for ca. 97% of total variability and separates cluster III from clusters I/II (F-test value = 104.07; degrees of freedom, DF = 4 and 28; p < 0.0001), due to the high content of selina-1,3,7(11)-trien-8-one. On the other hand, the second discriminant function (F2) distinguishes cluster II from I as a result of the high content of curzerene (F = 37.07; DF = 1 and 15; p < 0.0001). In addition, the two discriminant functions allow an accurate prediction of total well-classification in the original clusters via cross-validation or Jackknife approaches. These approaches consider a slightly reduced number of samples from the parent data set, estimate parameters for each of these modified data sets, and then calculate the accuracy of the predictions for the samples previously removed by the resulting models.27
Similarly to a previous study which analyzed essential leaf oils of purple and yellow fruits of E. brasiliensis,23 our results may confirm the presence of varieties/chemotypes for E. uniflora due to the remarkable difference in chemical composition according to fruit colour biotypes. In fact, the literature combined with our findings led us to deduce that the chemotype growing in the Brazilian Northeast and in Nigeria, which exhibited selina-1,3,7(11)-trien-8-one and selina-1,3,7(11)-trien-8-one epoxide (cluster III), is probably the most common orange-red fruit biotype in this region.9,11 In recent study,28 the chemical polymorphism in leaf oils of this chemotype revealed to be environmentally determined by a clear seasonal influence. Two other essential oil chemotypes of the plant have been reported: the chemotype growing in the south of Brazil, which contained α- and β-selinene and nerolidol,21 and the chemotype growing in Argentina, whose main constituents were monoterpenes and the sesquiterpene nerolidol.20 These chemical data could be confirmed by genetic studies based on nuclear and chloroplastic DNA markers. These studies indicate that a more restricted gene flow may exist between E. uniflora populations from the Brazilian South, Northeast and Southeast.29
The chemical oil composition from the Brazilian Northern sampling site is similar to that of cluster II,10 although the content of germacrene B was higher than that of our samples. In addition, the abundance of curzerene (20.5%), germacrene B (21.6%), atractylone (11.7%) and germacrone (17.3%) in our samples (cluster I) showed a marked deviation from previous leaf oil investigations, despite the fact that this study has described higher contents of curzerene (50.2%);12 curzerene (19.7%), atractylone (16.9%) and selina-1,3,7(11)-trien-8-one (17.8%);11 curzerene (30%), germacrene B (15.6%) and germacrone (32.8%);10 curzerene (24%), selina-1,3,7(11)-trien-8-one (17%), selina-1,3,7(11)-trien-8-one epoxide (14%),8 and selina-1,3,7(11)-trien-8-one (48.5%).30 Furthermore, oil constituents such as pulegone, carvone, a-selinene and nerolidol, typical in other studies, were not detected in our oil samples.20 This variation pattern in the leaf essential oil suggests that the observed chemical variations may be a result of the existence of new chemotypes for E. uniflora associated to yellowish, dark red and purple fruit colour biotypes.
Representative clustered oils were investigated against a P. brasiliensis reference strain (ATCC 90659) and three clinical isolates (Pb65, Pb1578 and Pb8334) via the standard broth macrodilution method, following NCCL M27-A2 guidelines with modifications.31 Based on MIC and MFC values (Table 3), the most significant finding was obtained with the oil from cluster II. The yeast form of P. brasiliensis was completely inhibited at a concentration of 62.5 µg mL-1, whereas oils from clusters III and I exhibited MIC values of 250 and 500 µg mL-1, respectively. MIC values were similar against various isolates and in relation to those non-polar fractions of the previously reported leaf ethanolic extract.7 All three oils readily killed P. brasiliensis isolates, although the oil from cluster II had the lowest MFC. These results may be associated to curzerene and germacrone in cluster II. On the other hand, the inhibitions and fungicidal activities of oils from clusters III and I may be related to selinatrienone derivatives and curzerene, respectively. Curzerene is structurally related to atractylone, which has shown an antiproliferative effect on tumour cell lines.32 Its derivative, atractylenolide III, inhibited the synthesis of mammal heat shock proteins HSP60 and HSP27 kDa family and enhances the effect of chemotherapy.33 Germacrone and curzerene have been described as active phytoconstituents in rizhome oils from Curcuma zedoaria (Berg.) Rosc. (Zingiberaceae).34
Conclusions
The essential oil from E. uniflora leaves grown in the Central Brazilian Cerrado revealed a high polymorphism which might be influenced by fruit colour biotypes. Therefore, caution is required in all chemical and biological investigations of Eugenia oils because of such influence. In addition, E. uniflora essential oils showed very promising results in the antifungal assay against the systemic fungus P. brasiliensis and warrant further study.
Experimental
Plant material
E. uniflora leaves in the vegetative phenophase were collected in June 2002 and 2003 and from June to August 2007 in the vicinities of Goiânia (S 16° 35 14"; W 49° 17 40"; 736 m; four red fruit specimens), Santo Antônio de Goiás (S 16° 30; W 49° 19; 773 m; two yellowish and two purple specimens), Nova Veneza (S 16° 21 44"; W 49° 18 39"; 773 m; eight red fruit specimens) and Anápolis (S 16° 20 13"; W 48° 56 19"; 1034 m; two red fruit specimens), Goiás State, Brazil. Leaves from red fruit specimens were also separated in bright red (two samples), red-orange (five samples) and dark red (seven samples) biotypes. Fruit colour biotypes were based when the fruits were completely ripe. Specimens were identified by Professor Heleno Dias Ferreira of the Department of Biology of Universidade Federal de Goiás (UFG), Goiás State, Brazil. A voucher specimen is deposited at UFGs herbarium (code number 25477).
To assess the chemical oil composition, the leaves were dried at room temperature for 7 days at 30 °C until constant weight. After having been powdered, the dried phytomass (10-50 g) of each sample was submitted to hydrodistillation (2 h) using a modified Clevenger-type apparatus. At the end of each distillation the oils were collected and dried with anhydrous Na2SO4, transferred to glass flasks and kept at a temperature of -18 °C. Oil yields (%) were based on the dried weight of plant samples.
Chemical analysis
Oil sample analyses were performed on a GC-MS Shimadzu QP5050A instrument under the following conditions: a CBP-5 (Shimadzu) fused silica capillary column (30 m × 0.25 mm i.d., 0.25 mm film thickness) connected to a quadrupole detector operating in the EI mode at 70 eV with a scan mass range of 40-400 m/z at a sampling rate of 1.0 scan s-1; carrier gas: He (1 mL min-1); injector and interface temperatures of 220 °C and 240 °C, respectively, with a split ratio of 1:20. The injection volume was 0.4 µL (20% in hexane) and the oven temperature was raised from 60 to 246 °C, with an increase of 3 °C min-1, then of 10 °C min-1 to 270 °C, holding the final temperature for 5 min. Selected Ion Monitoring (SIM) was performed in the same conditions of the scan analyses. Individual components were identified by comparing their linear retention indexes (Rl),35 by co-injection with a C8-C32n-alkanes series,36 mass spectra with those of the literature,35 and computerised MS-database using NIST libraries.35
Chemical variability
Principal component analysis (PCA) was applied to examine the interrelationships between populations and their essential oil constituents, via Système Portable dAnalyse des Données-SPAD software package, version 5.5, Centre International de Statistique et dInformatique Appliquées, France (2002). Cluster analysis was also applied to the study of sample similarity on the basis of essential oil constituent distribution. Nearest neighbour complete linkage technique by Benzécri algorithm was used as an index of similarity and hierarchical clustering was performed according to Wards variance minimizing method.37,38 For the variable selection, the threshold of residual eigenvalues (≤ 0.70) in the original data matrix (18 samples × 36 variables = 648 data) was used to establish the maximum number of variables which could be removed. Effectively eliminated variables expressed the highest loadings in the lowest residual eigenvalues and also contributed ≤ 0.7% to the chemical profiles (average values).
Canonical Discriminant analysis via SAS CANDISC procedure (Statistical Analysis System, SAS Institute Inc., Cary, NC, 1996) was used to differentiate samples and clusters on the basis of oil composition. The predictive ability of canonical discriminant functions was evaluated by cross-validation and Jackknife approaches as implemented in SAS statistical package. Prior to the multivariate analysis, the data was preprocessed by auto-scaling and mean centering.
Average multiple comparisons were established by univariate variance analysis (ANOVA) using SAS GLM analyses. All data were checked for homoscedasticity with Hartleys test. This test revealed significant deviation from the basic assumption for the oil components caryophyllene oxide (Table 1), allo-aromadendrene, d-selinene, germacrene A, spathulenol, germacrone and sesquiterpene hydrocarbons, which were arcsine and rank-transformed, respectively. Whenever a difference was established, a post-hoc Tukey test was performed. Results are shown as mean values and are joined by the standard deviation of independent measurements in some cases. P-values below 0.05 were regarded as significant.
Microorganisms
Yeast cells of a reference strain (ATCC 90659) and of three clinical isolates of P. brasiliensis recovered from patients with paracoccidioidomycosis were subcultured every seven days on agar Sabouraud medium (1% peptone, 0.5% yeast extract, 0.1% brain heart infusion; 4% glucose and 0.8% agar, pH 7.2) at 36 ± 1 °C prior to the susceptibility testing.
Antifungal susceptibility test
Susceptibility testing was performed following the standard broth dilution method according to NCCLS-CLSI M27-A2 guidelines with modifications.31 Briefly, for the inhibition assays, yeast cells in their exponential growth phase were kept on solid RPMI 1640 with glutamine and phenol red, supplemented with 0.2% glucose and buffered to a pH 7.0 with 0.165 mol L-1 3-morpholinepropanesulfonic acid (MOPS; Sigma, St. Louis) for seven days at 36 ± 1 °C. Sterile stock solutions of representative clustered oils from E. uniflora leaves were freshly prepared in DMSO. Serial twofold dilutions from stock solutions were prepared with sterile RPMI 1640 medium as the diluent to yield final essential oil concentrations ranging from 62.5 to 500 µg mL-1. Essential oils-free controls tubes were included. Inocula concentrations were determined spectrophotometrically via a yeast suspension in sterile 0.85% NaCl adjusted to 85% transmittance at 520 nm. The mixture was vortexed to disperse aggregated cells density. Aliquots of 0.1 mL of this suspension were added to 2.4 mL of broth RPMI 1640 containing the essential oil dilutions. This mixture was incubated at 36 °C in shaker at 120 rpm for 14 days and the transmittance was measured at 520 nm every 48 h. The minimal inhibition concentration (MIC) was spectrophotometrically determined after the wells had been thoroughly mixed to produce a homogeneous suspension. MIC was defined as the lowest concentration at which the optical density (OD) was reduced to ≤ 50% of the OD of the growth control well after 8-10 days of incubation.39 Subsequently, the minimal fungicide concentration (MFC) was determined by the reinoculum of cultures in RPMI 1640 agar medium. MFC was defined as the lowest oil concentration which completely inhibited yeast growth after 10 days of incubation at 36 °C. Duplicates were maintained for each concentration.
Supplementary Information
GC-MS chromatograms of representative clustered oils from E. uniflora leaves with different fruit colour biotypes are available free of charge at http://jbcs.sbq.org.br as PDF file.
Acknowledgments
We are indebted to CNPq, PADCT III and FUNAPE/UFG for financial support; and to CAPES for the fellowship granted to D.P.C. We also thank Prof. Dr. Heleno D. Ferreira of the School of Biology-UFG for the botanical identification and Profa. Dra. Maria Gizelda O. Tavares of the Institute of Chemistry-UFG for the leaf sample from bright red fruit biotype.
References
1. Lupi, O.; Tyring, S. K.; McGinnis, M. R.; J. Am. Acad. Dermatol. 2005, 53, 931.
2. Marques, S. A.; Robles, A. M.; Tortorano, A. M.; Tuculet, M. A.; Negroni, R.; Mendes, R. P.; Med. Mycol. 2000, 38, 269; Santo A. H.; Rev. Panam. Salud Publica 2008, 23, 313.
3. Restrepo, A. In Principles and Practice of Infectious Diseases; Mandel, G. I.; Bennet, J. F.; Dolin, R., eds.; Philadelphia: Churchill Livingstone, 2000.
4. Nicola, A. M.; Andrade, R. V.; Silva-Pereira, I.; Genet. Mol. Res. 2005, 4, 346.
5. Neckers, L.; Tatu, U.; Cell Host Microbe 2008, 4, 519.
6. Lima, M. M.; MSc Dissertation, Universidade Federal de Goiás, Brazil, 2001; Santos, G. D.; Ferri, P. H.; Santos, S. C.; Bao, S. B.; Soares, C. M. A.; Pereira, M.; Med. Mycol. 2007, 45, 609.
7. Santos, S. C.; Ferri, P. H.; Ribeiro, J. P; Guimarães, D. O.; Silva, M. O.; Garcia, A. C. F.; Pires, J. S.; Castro, A. C. M.; Silva, M. R. R.; Paula, J. R.; Rev. Bras. Pl. Med. 2004, 7, 30.
8. Weyerstahl, P.; Marschall-Weyerstahl, H.; Christiansen, C.; Oguntimien, B. O.; Adeoye, A. O.; Planta Med. 1988, 54, 546.
9. Morais, S. M.; Craveiro, A. A.; Machado, M. I. L.; Alencar, J. W.; Mattos, F. J. A.; J. Essent. Oil Res. 1996, 8, 449.
10. Maia, J. G. S.; Andrade, M. H. L.; Silva, M. H. L.; Zoghbi, M. G. B.; J. Essent. Oil Res. 1999, 11, 727.
11. Ogunwande, I. A.; Olawore, N. O.; Ekundayo, O.; Walker, T. M.; Schmidt, J. M.; Setzer, W. N.; Int. J. Aromath. 2005, 15, 147.
12. Melo, R. M.; Corrêa, V. F. S.; Amorim, A. C. L.; Miranda, A. L. P.; Rezende, C. M.; J. Braz. Chem. Soc. 2007, 18, 179.
13. Schapoval, E. E. S.; Silveira, S. M.; Miranda, M. L.; Alice, C. B.; Henriques, A. T.; J. Ethnopharmacol. 1994, 44, 137.
14. Almeida, C. E.; Karnikowski, M. G. O.; Foleto, R.; Baldisserotto, B.; Rev. Saude Publica 1995, 29, 428; Ferro, E.; Schinini, A.; Maldonado, M.; Rosner, J.; Hirschmann, G. S.; J. Ethnopharmacol. 1988, 24, 321.
15. Arai, J.; Amagaya, S.; Komatsu, Y.; Okuda, M.; Mayashi, T.; Kasai, M.; Arisawa, M.; Momose, Y.; J. Ethnopharmacol. 1999, 68, 307; Matsumura, T.; Kasai, M.; Hayashi, T.; Arisawa, M.; Momose, Y.; Arai, I.; Amagaya, S.; Omatsu, Y.; Pharm. Biol. 2000, 38, 302; Morioka, K.; Nojima, H.; Kurosaki, F.; Arisawa, M.; Kuraishi, Y.; Momose, Y.; Phytomedicine 2000, 7, 99.
16. Agbedahunsi, J. M.; Aladesanmi, A. J.; Fitoterapia 1993, 64, 174; Consolini, A. E.; Baldini, O. A. N.; Amat, A. G.; J. Ethnopharmacol. 1999, 66, 33.
17. Wazlawik, E.; Silva, M. A.; Peters, R. R.; Correia, J. F. G.; Farias, M. R.; Calixto, J. B.; Ribeiro do Valle, R. M.; J. Pharm. Pharmacol. 1997, 49, 433.
18. Adebajo A. C.; Oloke K. J.; Aladesanmi A. J.; Fitoterapia 1989, 60, 451; Fadeyi, M. O.; Akpan, U. E.; Phytother. Res. 1989, 3, 154; Lima, E. O.; Gompertz, O. F.; Giesbrecht, A. M.; Paulo, M. Q.; Mycoses 1993, 36, 333; Lee, M.-H.; Nishimoto, S.; Yang, L.-L.; Yen, K.-Y.; Hatano, T.; Yoshida, T.; Okuda, T.; Phytochemistry 1997, 44, 1343; Souza, L. K. H.; Oliveira, C. M. A.; Ferri, P. H.; Santos, S. C.; Oliveira Júnior, G. G.; Miranda, A. T. B.; Lião, L. M.; Silva, M. R. R.; Braz. J. Microbiol. 2002, 33, 247.
19. Lima, V. L. A. G.; Mélo, E. A.; Lima, D. E. S.; Sci. Agric. 2002, 59, 447; Sanchotene, M. C. C.; Frutíferas Nativas Úteis à Fauna na Arborização Urbana, 2ª ed., Sagra: Porto Alegre, 1989; Lederman, I. E.; Bezerra, J. E. F.; Calado, G.; A Pitangueira em Pernambuco, Secretaria de Agricultura, Empresa Pernambucana de Pesquisa Agropecuária: Recife, 1992; Franzon, R. C.; MSc Dissertation, Universidade Federal de Pelotas, 2004.
20. Viana, M. E. L.; Retamar, J. A.; An. Soc. Cient. Argent. 1971, 192, 111; Ubiergo, G.; Taher, H. A.; Talenti, E. C.; An. Asoc. Quim. Argent. 1987, 75, 377.
21. Henriques, A. T.; Sobral, M. E.; Cauduro, A. D.; Schapoval, E. E. S.; Bassini, V. L.; Lamaty, G.; Menut, C.; Bessière, J. M.; J. Essent. Oil Res. 1993, 5, 501.
22. Rücker, G.; Brasil e Silva, G. A.; Bauer, L.; Schikarski, M.; Plant Med. 1977, 31, 322; Pino, J. A.; Bello, A.; Urquiola, A.; Aguero, J.; Marbot, R.; J. Essent. Oil Res. 2003, 15, 70; Oliveira, A. L.; Lopes, R. B.; Cabral, F. A.; Eberlin, M. N.; Food Chem. 2006, 99, 1.
23. Moreno, P. R. H.; Lima, M. E. L.; Sobral, M.; Young, M. C. M.; Cordeiro, I.; Apel, M. A.; Limberger, R. P.; Henriques, A. T.; Sci. Agric. 2007, 64, 428.
24. Bezerra, J. E. F.; Silva Júnior, J. F.; Lenderman, I. E.; Pitanga (Eugenia uniflora L.), Série Frutas Nativas 1, Funep: Jaboticabal, 2000; Núcleo de Estudo em Fruticultura no Cerrado, http://www.fruticultura.iciag.ufu.br/pitangueira.html, acessed in March, 2009; Raseira, M. C. B.; Raseira, A. In Documentos 171; Antunes, L. E. C.; Raseira, M. C. B., eds., Embrapa Clima Temperado: Pelotas, 2006.
25. Sneath, P. H.; Sokal, R. R.; Principles of Numerical Taxonomy. A series of Books in Biology, W.H. Freeman: San Francisco, 1963.
26. Lebart, L.; Morineau, A.; Warwick, K. M.; Multivariate Descriptive Statistical Analysis. Correspondence Analysis and Related Techiniques for Large Matrices, John Wiley & Sons: New York, NY, 1984, ch. 4.
27. Wold, A.; Eriksson, L. In Chemometric Methods in Molecular Design, vol. 2; Waterbeemd, H., ed.; VCH: Weinheim, 1995, ch. 5.
28. Costa, D. P.; Santos, S. C.; Seraphin, J. C.; Ferri, P. H.; J. Braz. Chem. Soc. 2009, 20, 1287.
29. Salgueiro, F.; Felix, D.; Caldas, J. F.; Margis-Pinheiro, M.; Margis, R.; Diversity Distrib. 2004, 10, 201.
30. El-Shabrawy A. O.; Bull. Fac. Pharm. Cairo Univ. 1995, 33, 17.
31. National Committee for Clinical Laboratory Standards; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard, 2nd ed., M27-A2.
32. Wang, C. C.; Chen, L. G.; Yang, L. L.; Planta Med. 2002, 68, 204.
33. Yoichi, S.; Tomoko, T.; Toshimi, S.; Masayoshi, M.; Chikao, Y.; Kureha Chem., JP 10045585-A, 1998.
34. Lobo, R.; Prabhua, K. S.; Shirwaikar, A.; Shirwaikar, A.; J. Pharm. Pharmacol. 2009, 61, 13.
35. Adams, R. P.; Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed., Allured: Illinois, 2007; National Institute of Standards and Technology; PC version of the NIST/EPA/NIH Mass Spectral Database, U.S. Department of Commerce: Gaithersburg, 1998.
36. Van Den Dool, H.; Kratz, P. D. J. A.; J. Chromatogr. 1963, 11, 463.
37. Benzécri, J. -P.; L´Analyse des Données: la Taxinomie, Tome 1, Dunod: Paris, 1980.
38. Ward, J. H.; J. Am. Stat. Assoc. 1963, 58, 238.
39. Stevens, A. A.; Aristizabal, B. H.; Diagn. Microbiol. Infect. Dis. 1997, 29, 103.
Received: April 17, 2009
Web Release Date: February 11, 2010
Supplementary Information
- 1. Lupi, O.; Tyring, S. K.; McGinnis, M. R.; J. Am. Acad. Dermatol. 2005, 53, 931.
- 2. Marques, S. A.; Robles, A. M.; Tortorano, A. M.; Tuculet, M. A.; Negroni, R.; Mendes, R. P.; Med. Mycol. 2000, 38, 269;
- Santo A. H.; Rev. Panam. Salud Publica 2008, 23, 313.
- 3. Restrepo, A. In Principles and Practice of Infectious Diseases; Mandel, G. I.; Bennet, J. F.; Dolin, R., eds.; Philadelphia: Churchill Livingstone, 2000.
- 4. Nicola, A. M.; Andrade, R. V.; Silva-Pereira, I.; Genet. Mol. Res. 2005, 4, 346.
- 5. Neckers, L.; Tatu, U.; Cell Host Microbe 2008, 4, 519.
- 6. Lima, M. M.; MSc Dissertation, Universidade Federal de Goiás, Brazil, 2001;
- Santos, G. D.; Ferri, P. H.; Santos, S. C.; Bao, S. B.; Soares, C. M. A.; Pereira, M.; Med. Mycol. 2007, 45, 609.
- 7. Santos, S. C.; Ferri, P. H.; Ribeiro, J. P; Guimarães, D. O.; Silva, M. O.; Garcia, A. C. F.; Pires, J. S.; Castro, A. C. M.; Silva, M. R. R.; Paula, J. R.; Rev. Bras. Pl. Med. 2004, 7, 30.
- 8. Weyerstahl, P.; Marschall-Weyerstahl, H.; Christiansen, C.; Oguntimien, B. O.; Adeoye, A. O.; Planta Med. 1988, 54, 546.
- 9. Morais, S. M.; Craveiro, A. A.; Machado, M. I. L.; Alencar, J. W.; Mattos, F. J. A.; J. Essent. Oil Res. 1996, 8, 449.
- 10. Maia, J. G. S.; Andrade, M. H. L.; Silva, M. H. L.; Zoghbi, M. G. B.; J. Essent. Oil Res. 1999, 11, 727.
- 11. Ogunwande, I. A.; Olawore, N. O.; Ekundayo, O.; Walker, T. M.; Schmidt, J. M.; Setzer, W. N.; Int. J. Aromath. 2005, 15, 147.
- 12. Melo, R. M.; Corrêa, V. F. S.; Amorim, A. C. L.; Miranda, A. L. P.; Rezende, C. M.; J. Braz. Chem. Soc. 2007, 18, 179.
- 13. Schapoval, E. E. S.; Silveira, S. M.; Miranda, M. L.; Alice, C. B.; Henriques, A. T.; J. Ethnopharmacol. 1994, 44, 137.
- 14. Almeida, C. E.; Karnikowski, M. G. O.; Foleto, R.; Baldisserotto, B.; Rev. Saude Publica 1995, 29, 428;
- Ferro, E.; Schinini, A.; Maldonado, M.; Rosner, J.; Hirschmann, G. S.; J. Ethnopharmacol. 1988, 24, 321.
- 15. Arai, J.; Amagaya, S.; Komatsu, Y.; Okuda, M.; Mayashi, T.; Kasai, M.; Arisawa, M.; Momose, Y.; J. Ethnopharmacol. 1999, 68, 307;
- Matsumura, T.; Kasai, M.; Hayashi, T.; Arisawa, M.; Momose, Y.; Arai, I.; Amagaya, S.; Omatsu, Y.; Pharm. Biol. 2000, 38, 302;
- Morioka, K.; Nojima, H.; Kurosaki, F.; Arisawa, M.; Kuraishi, Y.; Momose, Y.; Phytomedicine 2000, 7, 99.
- 16. Agbedahunsi, J. M.; Aladesanmi, A. J.; Fitoterapia 1993, 64, 174;
- Consolini, A. E.; Baldini, O. A. N.; Amat, A. G.; J. Ethnopharmacol. 1999, 66, 33.
- 17. Wazlawik, E.; Silva, M. A.; Peters, R. R.; Correia, J. F. G.; Farias, M. R.; Calixto, J. B.; Ribeiro do Valle, R. M.; J. Pharm. Pharmacol. 1997, 49, 433.
- 18. Adebajo A. C.; Oloke K. J.; Aladesanmi A. J.; Fitoterapia 1989, 60, 451;
- Fadeyi, M. O.; Akpan, U. E.; Phytother. Res. 1989, 3, 154;
- Lima, E. O.; Gompertz, O. F.; Giesbrecht, A. M.; Paulo, M. Q.; Mycoses 1993, 36, 333;
- Lee, M.-H.; Nishimoto, S.; Yang, L.-L.; Yen, K.-Y.; Hatano, T.; Yoshida, T.; Okuda, T.; Phytochemistry 1997, 44, 1343;
- Souza, L. K. H.; Oliveira, C. M. A.; Ferri, P. H.; Santos, S. C.; Oliveira Júnior, G. G.; Miranda, A. T. B.; Lião, L. M.; Silva, M. R. R.; Braz. J. Microbiol. 2002, 33, 247.
- 19. Lima, V. L. A. G.; Mélo, E. A.; Lima, D. E. S.; Sci. Agric. 2002, 59, 447;
- Sanchotene, M. C. C.; Frutíferas Nativas Úteis à Fauna na Arborização Urbana, 2Ş ed., Sagra: Porto Alegre, 1989;
- Lederman, I. E.; Bezerra, J. E. F.; Calado, G.; A Pitangueira em Pernambuco, Secretaria de Agricultura, Empresa Pernambucana de Pesquisa Agropecuária: Recife, 1992;
- Franzon, R. C.; MSc Dissertation, Universidade Federal de Pelotas, 2004.
- 20. Viana, M. E. L.; Retamar, J. A.; An. Soc. Cient. Argent. 1971, 192, 111;
- Ubiergo, G.; Taher, H. A.; Talenti, E. C.; An. Asoc. Quim. Argent. 1987, 75, 377.
- 21. Henriques, A. T.; Sobral, M. E.; Cauduro, A. D.; Schapoval, E. E. S.; Bassini, V. L.; Lamaty, G.; Menut, C.; Bessière, J. M.; J. Essent. Oil Res. 1993, 5, 501.
- 22. Rücker, G.; Brasil e Silva, G. A.; Bauer, L.; Schikarski, M.; Plant Med. 1977, 31, 322;
- Pino, J. A.; Bello, A.; Urquiola, A.; Aguero, J.; Marbot, R.; J. Essent. Oil Res. 2003, 15, 70;
- Oliveira, A. L.; Lopes, R. B.; Cabral, F. A.; Eberlin, M. N.; Food Chem. 2006, 99, 1.
- 23. Moreno, P. R. H.; Lima, M. E. L.; Sobral, M.; Young, M. C. M.; Cordeiro, I.; Apel, M. A.; Limberger, R. P.; Henriques, A. T.; Sci. Agric. 2007, 64, 428.
- 24. Bezerra, J. E. F.; Silva Júnior, J. F.; Lenderman, I. E.; Pitanga (Eugenia uniflora L.), Série Frutas Nativas 1, Funep: Jaboticabal, 2000; Núcleo de Estudo em Fruticultura no Cerrado, http://www.fruticultura.iciag.ufu.br/pitangueira.html, acessed in March, 2009;
- Raseira, M. C. B.; Raseira, A. In Documentos 171; Antunes, L. E. C.; Raseira, M. C. B., eds., Embrapa Clima Temperado: Pelotas, 2006.
- 25. Sneath, P. H.; Sokal, R. R.; Principles of Numerical Taxonomy A series of Books in Biology, W.H. Freeman: San Francisco, 1963.
- 26. Lebart, L.; Morineau, A.; Warwick, K. M.; Multivariate Descriptive Statistical Analysis. Correspondence Analysis and Related Techiniques for Large Matrices, John Wiley & Sons: New York, NY, 1984, ch. 4.
- 27. Wold, A.; Eriksson, L. In Chemometric Methods in Molecular Design, vol. 2; Waterbeemd, H., ed.; VCH: Weinheim, 1995, ch. 5.
- 28. Costa, D. P.; Santos, S. C.; Seraphin, J. C.; Ferri, P. H.; J. Braz. Chem. Soc. 2009, 20, 1287.
- 29. Salgueiro, F.; Felix, D.; Caldas, J. F.; Margis-Pinheiro, M.; Margis, R.; Diversity Distrib. 2004, 10, 201.
- 30. El-Shabrawy A. O.; Bull. Fac. Pharm. Cairo Univ. 1995, 33, 17.
- 31. National Committee for Clinical Laboratory Standards; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts Approved Standard, 2nd ed., M27-A2.
- 32. Wang, C. C.; Chen, L. G.; Yang, L. L.; Planta Med. 2002, 68, 204.
- 33. Yoichi, S.; Tomoko, T.; Toshimi, S.; Masayoshi, M.; Chikao, Y.; Kureha Chem., JP 10045585-A, 1998
- 34. Lobo, R.; Prabhua, K. S.; Shirwaikar, A.; Shirwaikar, A.; J. Pharm. Pharmacol. 2009, 61, 13.
- 35. Adams, R. P.; Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed., Allured: Illinois, 2007;
- National Institute of Standards and Technology; PC version of the NIST/EPA/NIH Mass Spectral Database, U.S. Department of Commerce: Gaithersburg, 1998.
- 36. Van Den Dool, H.; Kratz, P. D. J. A.; J. Chromatogr 1963, 11, 463.
- 37. Benzécri, J. -P.; L´Analyse des Données: la Taxinomie, Tome 1, Dunod: Paris, 1980.
- 38. Ward, J. H.; J. Am. Stat. Assoc 1963, 58, 238.
- 39. Stevens, A. A.; Aristizabal, B. H.; Diagn. Microbiol. Infect. Dis. 1997, 29, 103.
Publication Dates
-
Publication in this collection
13 July 2010 -
Date of issue
2010
History
-
Accepted
11 Feb 2010 -
Received
17 Apr 2009