Abstract
Objective:
Roux-en-Y gastric bypass (RYGB) reduces body weight and the comorbidities associated with obesity. The aim of this study was to evaluate whether glucose and lipid profiles were maintained during a 5-year follow-up period after RYGB.
Subjects and methods:
Anthropometric and laboratory data from 323 patients who had undergone this operation were analyzed. Differences in laboratory variables between the baseline and 12, 24, 36, 48 and 60 months postoperatively (PO) were assessed using a one-way ANOVA test to compare the three groups. Delta significance using one-way ANOVA was performed to assess anthropometric variable in the postoperative period (p < 0.05).
Results:
77 patients (24%) were included in Group 1 (G1), 101 (32%) in Group 2 (G2), and 141 (44%) in Group 3 (G3). The majority of patients, 71.7% in G1, 82.8% in G2, and 70% in G3, showed high triglycerides (TG) before surgery. A decrease in weight loss was observed in all groups followed by an increase in body weight in G2 and G3 at 36, 48 and 60 months. Laboratory results for G1, G2 and G3 showed no significant differences between groups at baseline and during the post-operative period.
Conclusion:
Our results suggest that weight regain after RYGB has no significant impact on the long-term evolution of the lipid profile and glycemia.
Keywords
Bariatric surgery; weight regain; lipemia; glycemia
INTRODUCTION
Obesity is considered a risk factor for the development of various comorbidities, including impaired glucose homeostasis and cardiovascular disease (11. Stefater M, Kohli R, Inge T. Advances in the surgical treatment of morbid obesity. Mol Aspects Med. 2013;34(1):84-94.). Roux-en-Y gastric bypass (RYGB) is the most commonly performed surgical treatment for obesity; however, although it is well documented that RYGB results in weight loss and metabolic improvements, some degree of weight regain has been observed 2–3 years after surgery (22. Christou N, Look D, Maclean L. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244(5):734-40.), supporting the hypothesis that obesity is a chronic, incurable and progressive disease (33. Malinowski S. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci. 2006;331(4):219-25.).
RYGB includes the anatomical diversion of the upper gastrointestinal tract. These changes in intestinal anatomy are thought to alter the function of the nutrient-driven enteroendocrine system, causing beneficial effects on glucose homeostasis that are independent of weight loss itself (44. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248-256.e5.). In addition, weight loss elicits metabolic and cardiovascular benefits after RYGB, but evidence suggests that surgery might also activate weight-independent lipid amelioration mechanisms (11. Stefater M, Kohli R, Inge T. Advances in the surgical treatment of morbid obesity. Mol Aspects Med. 2013;34(1):84-94.). Garcia-Marirrodriga and cols. (55. Garcia-Marirrodriga I, Amaya-Romero C, Ruiz-Diaz GP, Férnandez S, Ballesta-López C, Pou JM, et al. Evolution of lipid profiles after bariatric surgery. Obes Surg. 2012;22(4):609-16.) reported significant associations between lipid subfractions and excess weight loss after RYGB. However, a recent study conducted by Bradley and cols. (66. Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest. 2012;122(12):4667-74.) does not support the notion of clinically meaningful, weight loss-independent effects of RYGB surgery on β cell function and insulin sensitivity in non diabetic obese subjects after they have experienced considerable weight loss.
Weight loss results in improvements in glycemia and lipemia, and this is most evident in patients who have undergone RYGB (77. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003 Oct;238(4):467-84.). However, an elegant review by Shah and cols. (88. Shah M1, Simha V, Garg A. Review: Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91(11):4223-31.) showed that the initial improvements seen following surgery decreased over a long-term follow-up period, possibly because of weight regain or unsatisfactory weight loss. Since weight gain can be considered a risk factor for dyslipidemia and changes in glucose levels, the aim of this study was to evaluate whether glucose and lipid profiles were maintained during a 5-year follow-up period after RYGB.
SUBJECTS AND METHODS
Subjects
This retrospective study was conducted by examining the medical records of 323 patients who underwent RYGB in a private clinic in Rio de Janeiro from 2000 to 2007 and maintained regular postoperative follow-up visits for at least 24 months after surgery. Personal, anthropometric and laboratory data were obtained from the medical records. This study was approved by the Research and Ethics Committee of the Clementino Fraga Filho University Hospital (176/09).
Three groups were classified according to the rate of inclination of each individual weight over time using linear regression slope for subjects with at least 36 months follow-up. The slope was obtained from the first 24 months after surgery, when patients’ body weight is expected to stabilize. Negative slopes were considered G1 (patients without weight regain). Positive slopes (variation between 0 and 2 kg) were considered G2 (patients who might or might not regain weight). Positive slopes (over to 2 kg) were considered G3 (patients with weight regain).
Anthropometric and laboratory assessment
Anthropometric variables which were assessed included height and weight measured during the preoperative period and at 12, 24, 36, 48 and 60 months PO. Body mass index (BMI) was calculated by dividing the body weight in kilograms (kg) by the square of the height in meters (m2) (99. World Health Organization: Physical Status: the use and interpretation of anthropometry: Technical report series 854. Geneva; 1998. p. 1-452.). Total body mass and stature were measured following Gibson, using a mechanical anthropometric balance (Welmy®) with a maximum capacity of 300 kg, divided by 100 g, and the stadiometer from the anthropometric balance with a scale of 0.1 cm (1010. Gibson S. Principles of nutritional dymatic. New York: Oxford; 1990. p. 691.). The patients were barefoot and wore minimal clothing when weighed.
Laboratory parameters assessed included total cholesterol (TC), low-density lipoprotein cholesterol (LDLc), high-density lipoprotein cholesterol (HDLc), triglycerides (TG) and glucose. These were measured during the preoperative period and at 12, 24, 36, 48 and 60 months PO. TC, HDLc, TG and glucose were determined by the enzymatic-colorimetric method (1111. McGowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983;29(3):538-42.). Concentrations of LDLc were calculated based on Friedwald's equation (1212. Friedwald W, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502.). Concentrations of serum lipids that were considered normal were those provided in the guidelines of the National Cholesterol Education Program – Adult Treatment Panel III (NCEP/ATPIII): CT < 200 mg/dL, TG < 150 mg/dL, HDLc > 40 mg/dL e LDL-c < 130 mg/dL (1313. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-97.). A concentration of < 100 mg/dL was considered normal for fasting blood glucose (1414. American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35:11-63.).
Statistical analysis
Means and standard deviations (SD) were determined for quantitative data. All data were assessed for normality using the Kolmogorov-Smirnov test. The Chi-square test was used for the comparison of proportions of abnormal values in TC, LDLc, HDLc, TG and glucose to baseline. Differences in laboratory variables between the baseline and at 12, 24, 36, 48 and 60 months PO were assessed using a one-way ANOVA test to compare the groups. Delta significance using one-way ANOVA was used to assess anthropometric variables PO. Statistical significance was defined as two-tailed p < 0.05. Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) software, version 21.0 for Windows.
RESULTS
Of the 323 patients included in this study, 77 were included in G1 (24%), 101 in G2 (32%), and 141 in G3 (44%). Demographic data and the proportion of patients with abnormal laboratory values at baseline are shown in Table 1.
Table 2 showed no difference in the proportion of groups with abnormal values for the lipid subtractions and glucose concentration at baseline. Most patients from the 3 groups showed high TG before surgery.
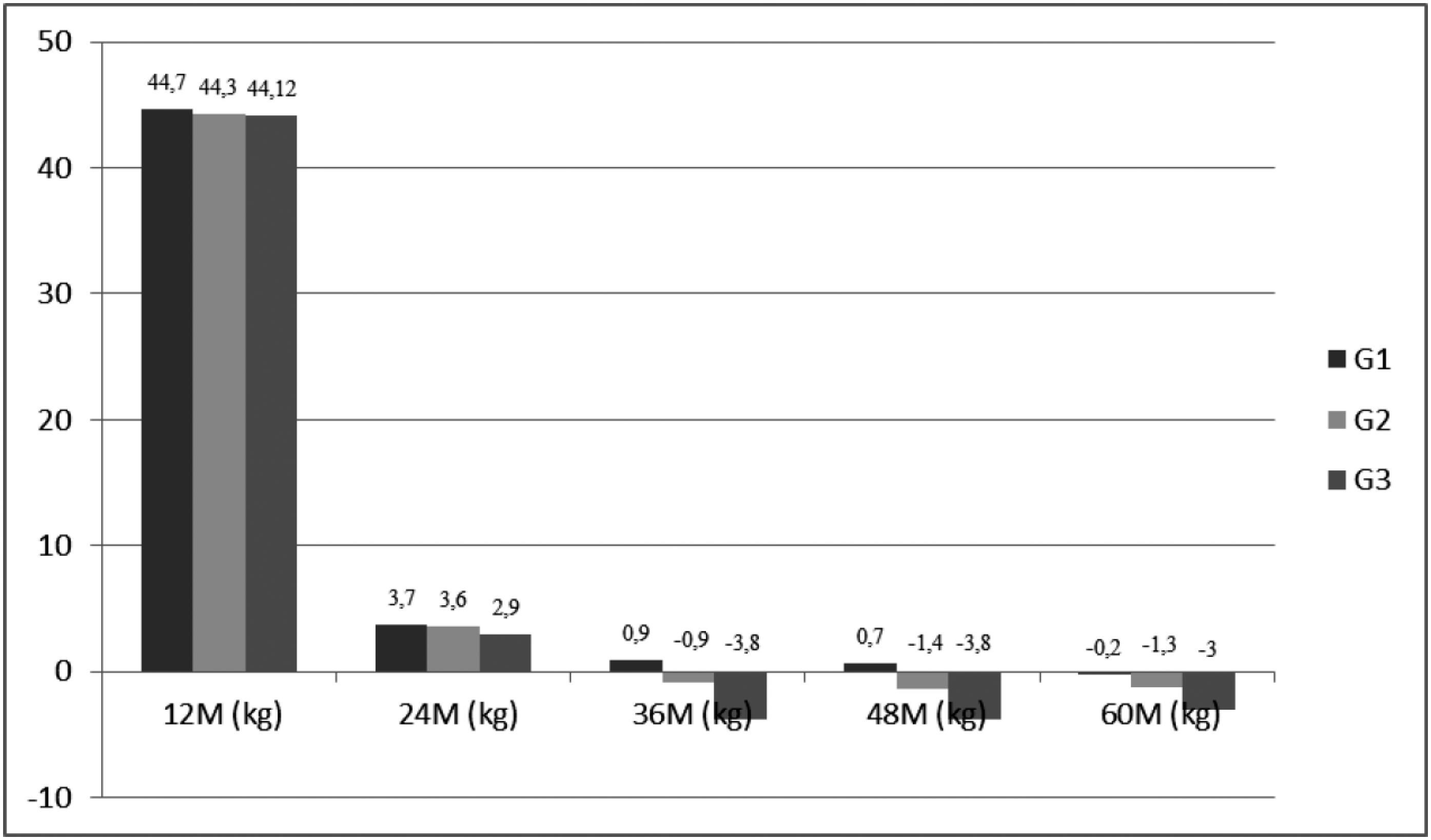
Figure 1 shows weight loss for G1, G2 and G3 during the postoperative period. Comparing these groups, a decrease in weight loss was observed in all groups followed by an increase in body weight for G2 and G3 at 36, 48, and 60 months (p < 0.01).
Weight loss (mean) in kilograms per year in patients who underwent RYGB.
M: months; kg: kilograms; G1: group 1; G2: group 2; G3: group 3.
Laboratory results for G1, G2 and G3 are shown in Table 3. No differences between groups at baseline and during the post-operative period were found.
Lipid profiles and glucose concentrations (means and standard deviation) of patients who underwent RYGB
DISCUSSION
This study examines the impact of weight regain on plasma glucose concentrations and lipid profiles in obese subjects during a 5-year follow-up period after marked RYGB-induced weight loss. The three groups of patients were comparable in terms of age, gender, anthropometric characteristics and laboratory results at baseline.
After the second or third year PO, long-term studies have shown a tendency for a weight increase in gastric bypass patients (1515. Dalcanale L, Oliveira CP, Faintuch J, Nogueira MA, Rondó P, Lima VM, et al. Long-term nutritional outcome after gastric bypass. Obes Surg. 2010;20(2):181-7.
16. Faria SL, de Oliveira Kelly E, Lins RD, Faria OP. Nutritional management of weight regain after bariatric surgery. Obes Surg. 2010;20(2):135-9.
17. Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: a significant 20% complication related to PYY. Nutrition. 2008;24(9):832-42.
18. Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008 Nov;93(11 Suppl 1):S89-96.
19. Lopez P, Patel N, Koche L. Outpatient complications encountered following Roux-en-Y gastric bypass. Med Clin North Am. 2007;91(3):471-83, xii.-2020. Christou N, Look D, Maclean L. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244(5):734-40.). In this study, weight loss did not differ significantly over the first 24 months after surgery.
Weight reduction can improve the lipid profile, alter carbohydrate metabolism, reduce morbidity and mortality, and generally enhance the quality of life of morbidly obese patients (2121. Francischi R, Pereira L, Freitas C, Klopfer M, Santos RC, Vieira P, et al. Obesidade: atualização sobre sua etiologia, morbidade e tratamento. Rev Nutr. 2000;13(1):17-28.). Upper gastrointestinal tract diversion results in weight loss, but also may have weight loss-independent metabolic effects. Studies have reported contradictory findings regarding weight-loss dependent and independent effects of gastric bypass on glucose homeostasis and lipid profiles (11. Stefater M, Kohli R, Inge T. Advances in the surgical treatment of morbid obesity. Mol Aspects Med. 2013;34(1):84-94.,88. Shah M1, Simha V, Garg A. Review: Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91(11):4223-31.).
The RYGB technique may itself improve total cholesterol and LDLc levels (2222. Benaiges D, Goday A, Ramon JM, Hernandez E, Pera M, Cano JF; Obemar Group. Laparoscopic sleeve gastrectomy and laparoscopic gastric bypass are equally effective for reduction of cardiovascular risk in severely obese patients at one year of follow-up. Surg Obes Relat Dis. 2011;7(5):575-80.). An observational study in obese patients confirmed that BMI is correlated with levels of TG and HDLc, but not with LDLc levels (2323. Sundquist J, Winkleby M, Pudaric S. Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988-1994. Third National Health and Nutrition Examination Survey. Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2001;49(2):109-16.). In our study, glucose and lipid profiles were lower in all groups after surgery and this was not related to weight. Further studies analyzing laboratory parameters and weight dynamics after RYGB might contribute to clarifying whether the metabolic improvements seen after RYGB rely on weight loss or are an effect of the surgical technique itself.
The proportion of patients with abnormal total cholesterol concentrations prior to surgery was high in all groups but decreased during the long-term outcomes. Brolin and cols. (2424. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg. 2000;4(5):464-9.) found that the greatest postoperative reductions in cholesterol and triglycerides generally occur in patients with the highest preoperative elevations, which is in accordance with our results for TG. Previous studies have shown a decline in total cholesterol and TG concentrations, and an elevation in HDLc concentrations, during the PO period. These changes were sustained over a long period of time in those patients who kept lost weight off as well as in those who experienced weight regain over time. Jamal and cols. (2525. Jamal M, Wegner R, Heitshusen D, Liao J, Samuel I. Resolution of hyperlipidemia follows surgical weight loss in patients undergoing Roux-en-Y gastric bypass surgery: a 6-year analysis of data. Surg Obes Relat Dis. 2011;7(4):473-9.) reported that the amelioration of the overall lipid profile was sustained over a 6-year PO follow-up.
Laguna and cols also showed that TG and LDL-C levels decreased 30% with respect to preoperative levels, while HDL-C increased 97% over initial values. Both TC:HDL-C and TG:HDL-C ratios normalized after RYGB and values were sustained over the weight regain period until the end of the study (2626. Laguna S, Andrada P, Silva C, Rotellar F, Valenti V, Gil MJ, et al. Body weight-independent variations in HDL-cholesterol following gastric bypass. In: Anales del Sistema Sanitario De Navarra. Navas Tolosa 21, Pamplona, 31002, Spain: Gobierno de Navarra, 2016. p. 23-33.).
Bradley and cols. (66. Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest. 2012;122(12):4667-74.) support the idea that weight loss itself is primarily responsible for the clinical effects of RYGB on insulin sensitivity, β cell function, and oral glucose tolerance in non diabetic obese adults. On the other hand, Reed and cols. (2727. Reed MA, Pories WJ, Chapman W, Pender J, Bowden R, Barakat H, et al. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab. 2011;96(8):2525-31.) showed improved fasting insulin and glucose levels a week after RYGB, despite persistent insulin resistance. Peterli and cols. (2828. Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250(2):234-41.) also reported early and augmented insulin responses as early as a week PO, potentially mediating improved early glycemic control. In our study, improved blood glucose levels were maintained throughout the postoperative period, regardless of weight regain. This may be associated with the enteroendocrine effects of RYGB, and suggests that glucose homeostasis after RYGB is weight-independent. Postoperative reduction in the levels of gastric inhibitory protein (GIP), and enhanced production of the antidiabetic hormone glucagon-like peptide (GLP-1), may underlie the changes in glucose seen after surgery (2929. Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg. Surg Obes Relat Dis. 2007;3(6):597-601.). In addition, patients who participated in a prospective human study of gastric bypass had increased intestinal peptide YY (PYY) and GLP-1 responses, which were sustained even more than a year PO (3030. Pournaras DJ, Osborne A, Hawkins SC, Mahon D, Ghatei MA, Bloom SR, et al. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes Surg. 2010;20(1):56-60.).
Numerous other factors have been implicated as potential contributors to the metabolic improvement observed after bariatric surgery, including other intestinal gut hormones (GLP-2, PYY), ghrelin (an anorexic hormone secreted by the gastric fundus), adipokines, the increased energy expenditure following surgery, changes in the gut microbiome, and bile acid metabolism (3131. Corcelles R, Daigle CR, Schauer PR. Management of endocrine disease: metabolic effects of bariatric surgery. Eur J Endocrinol. 2016;174(1):R19-28.).
Weight regain after gastric bypass has been attributed to multiple factors, including dilation of the gastric reservoir and gastrojejunostomy site, increased calorie intake of high glycemic index carbohydrates and fats, intolerance to red meat, hormonal alterations resulting from the adaptive process, binge eating, alcohol and drug consumption (3232. Barhouch AS, Zardo M, Padoin AV, Colossi FG, Casagrande DS, Chatkin R, et al. Excess weight loss variation in late postoperative period of gastric bypass. Obes Surg. 2010;20(11):1479-83.,3333. Odom J, Zalesin KC, Washington TL, Miller WW, Hakmeh B, Zaremba DL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20(3):349-56.), difficulty promoting behavior changes, and lack of physical exercise. However, low adherence to follow-up with the multidisciplinary team PO is notoriously prevalent and problematic following bariatric surgery (3434. Silver HJ, Torquati A, Jensen GL, Richards WO. Weight, dietary and physical activity behaviors two years after gastric bypass. Obes Surg. 2006;16(7):859-64.). Wardé-Kamar and cols. (3535. Wardé-Kamar J, Rogers M, Flancbaum L, Laferrère B. Calorie intake and meal patterns up to 4 years after Roux-en-Y gastric bypass surgery. Obes Surg. 2004;14(8):1070-9.) suggest that such low adherence may occur due to underestimation of, or misinformation about, the surgery's consequences after long periods of time. Patients who regain weight may also feel embarrassed to seek help from the care service center where they had the operation. Reasons such as loss or change of health insurance, job loss, moving to another city or simply choosing not to continue the treatment after surgery may also reduce follow-up participation.
The limitations of our study include the retrospective nature of the analysis, incomplete follow-up, reduced sample size at the end of the study, and difficulty contacting patients who did not attend their scheduled follow-up appointments. These limitations are commonly reported in the literature for this kind of study. As laboratory results might change with further increases in body weight, a follow-up period of longer than 5 years would be necessary to establish whether they eventually return to preoperative levels (impairing the surgery's success and the patient's health). However, in spite of these limitations, long-term studies are crucial to determine long-term patient outcomes after bariatric surgery.
In conclusion, our results suggest that weight regain has no significant impact in the long-term evolution of the lipid profile and glycemia after RYGB. This study highlights the need for closer follow-up of RYGB patients, and for the implementation of actions aimed at improving adherence to the treatment protocols established by multidisciplinary teams. Additional long-term studies are required to establish whether a weight regain threshold might affect laboratory results, and to elucidate the exact mechanisms leading to these effects and their clinical consequences for the patient's health.
Acknowledgements:
we thank Fernando Luiz Barroso.
REFERENCES
-
1Stefater M, Kohli R, Inge T. Advances in the surgical treatment of morbid obesity. Mol Aspects Med. 2013;34(1):84-94.
-
2Christou N, Look D, Maclean L. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244(5):734-40.
-
3Malinowski S. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci. 2006;331(4):219-25.
-
4Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248-256.e5.
-
5Garcia-Marirrodriga I, Amaya-Romero C, Ruiz-Diaz GP, Férnandez S, Ballesta-López C, Pou JM, et al. Evolution of lipid profiles after bariatric surgery. Obes Surg. 2012;22(4):609-16.
-
6Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest. 2012;122(12):4667-74.
-
7Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003 Oct;238(4):467-84.
-
8Shah M1, Simha V, Garg A. Review: Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91(11):4223-31.
-
9World Health Organization: Physical Status: the use and interpretation of anthropometry: Technical report series 854. Geneva; 1998. p. 1-452.
-
10Gibson S. Principles of nutritional dymatic. New York: Oxford; 1990. p. 691.
-
11McGowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983;29(3):538-42.
-
12Friedwald W, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502.
-
13Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-97.
-
14American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35:11-63.
-
15Dalcanale L, Oliveira CP, Faintuch J, Nogueira MA, Rondó P, Lima VM, et al. Long-term nutritional outcome after gastric bypass. Obes Surg. 2010;20(2):181-7.
-
16Faria SL, de Oliveira Kelly E, Lins RD, Faria OP. Nutritional management of weight regain after bariatric surgery. Obes Surg. 2010;20(2):135-9.
-
17Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: a significant 20% complication related to PYY. Nutrition. 2008;24(9):832-42.
-
18Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008 Nov;93(11 Suppl 1):S89-96.
-
19Lopez P, Patel N, Koche L. Outpatient complications encountered following Roux-en-Y gastric bypass. Med Clin North Am. 2007;91(3):471-83, xii.
-
20Christou N, Look D, Maclean L. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244(5):734-40.
-
21Francischi R, Pereira L, Freitas C, Klopfer M, Santos RC, Vieira P, et al. Obesidade: atualização sobre sua etiologia, morbidade e tratamento. Rev Nutr. 2000;13(1):17-28.
-
22Benaiges D, Goday A, Ramon JM, Hernandez E, Pera M, Cano JF; Obemar Group. Laparoscopic sleeve gastrectomy and laparoscopic gastric bypass are equally effective for reduction of cardiovascular risk in severely obese patients at one year of follow-up. Surg Obes Relat Dis. 2011;7(5):575-80.
-
23Sundquist J, Winkleby M, Pudaric S. Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988-1994. Third National Health and Nutrition Examination Survey. Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2001;49(2):109-16.
-
24Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg. 2000;4(5):464-9.
-
25Jamal M, Wegner R, Heitshusen D, Liao J, Samuel I. Resolution of hyperlipidemia follows surgical weight loss in patients undergoing Roux-en-Y gastric bypass surgery: a 6-year analysis of data. Surg Obes Relat Dis. 2011;7(4):473-9.
-
26Laguna S, Andrada P, Silva C, Rotellar F, Valenti V, Gil MJ, et al. Body weight-independent variations in HDL-cholesterol following gastric bypass. In: Anales del Sistema Sanitario De Navarra. Navas Tolosa 21, Pamplona, 31002, Spain: Gobierno de Navarra, 2016. p. 23-33.
-
27Reed MA, Pories WJ, Chapman W, Pender J, Bowden R, Barakat H, et al. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab. 2011;96(8):2525-31.
-
28Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250(2):234-41.
-
29Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg. Surg Obes Relat Dis. 2007;3(6):597-601.
-
30Pournaras DJ, Osborne A, Hawkins SC, Mahon D, Ghatei MA, Bloom SR, et al. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes Surg. 2010;20(1):56-60.
-
31Corcelles R, Daigle CR, Schauer PR. Management of endocrine disease: metabolic effects of bariatric surgery. Eur J Endocrinol. 2016;174(1):R19-28.
-
32Barhouch AS, Zardo M, Padoin AV, Colossi FG, Casagrande DS, Chatkin R, et al. Excess weight loss variation in late postoperative period of gastric bypass. Obes Surg. 2010;20(11):1479-83.
-
33Odom J, Zalesin KC, Washington TL, Miller WW, Hakmeh B, Zaremba DL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20(3):349-56.
-
34Silver HJ, Torquati A, Jensen GL, Richards WO. Weight, dietary and physical activity behaviors two years after gastric bypass. Obes Surg. 2006;16(7):859-64.
-
35Wardé-Kamar J, Rogers M, Flancbaum L, Laferrère B. Calorie intake and meal patterns up to 4 years after Roux-en-Y gastric bypass surgery. Obes Surg. 2004;14(8):1070-9.
Publication Dates
-
Publication in this collection
17 May 2018 -
Date of issue
June 2018
History
-
Received
29 July 2017 -
Accepted
17 Feb 2018