ABSTRACT
The relationship between plants and frugivorous animals is modulated by morphological and nutritional characteristics of fruits, as well as their seasonal availability across habitats. We evaluated fruiting phenology, fruit morphology and nutritional characteristics of 35 abundant plant species from 15 families associated with frugivorous birds from distinct habitats in the Cerrado (savanna, forest, and palm swamp). For a subset of 16 plant species, we also evaluated the overlap in interactions with frugivorous birds using data from the literature. Open-habitat plants had their fruiting peak during the rainy season, while fruiting of forest species was evenly distributed across the year. Plants of the same family exhibited similar fruit morphology and nutritional characteristics. Most plants had fruits with more sugars than lipids, while all species with higher lipid content were from savanna habitats and produced fruits during the rainy season, the peak reproductive season for birds. Assemblages of frugivorous birds exhibited considerable overlap, irrespective of habitat or fruiting season of plants. The complementarity found among habitats, considering seasonal availability and nutritional profile of fruits for frugivorous birds, is relevant for community maintenance and regeneration. Therefore, this landscape level complexity should always be considered in conservation and restoration policies for the Cerrado.
Keywords:
Copaifera langsdorffii; Matayba guianensis; Miconia rubiginosa; Neotropical savanna; Ouratea; Rourea induta; Rudgea viburnoides; seed dispersal; Styrax ferrugineus; Vereda
Introduction
A large percentage of woody plants, from ca. 20 to 90 % depending on the community, rely on interactions with frugivorous animals for seed dispersal (Jordano 2014Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.). In turn, fleshy fruits are an important resource for animals, such as birds, which are the primary frugivores and seed dispersers in many communities (Galetti et al. 2011Galetti M, Pizo MA, Morellato LPC. 2011. Diversity of functional traits of fleshy fruits in a species-rich Atlantic rain forest. Biota Neotropica 11: 181-193.; Fleming & Kress 2011Fleming TH, Kress WJ. 2011. A brief history of fruits and frugivores. Acta Oecologica 37: 521-530.; Kuhlmann & Ribeiro 2016aKuhlmann M, Ribeiro JF. 2016a. Fruits and frugivores of the Brazilian Cerrado: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 495-507.). The relative importance of plants for frugivorous animals is strongly influenced by fruit characteristics and fruiting phenology (Fenner 1998Fenner M. 1998. The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, Evolution and Systematics 1: 78-91.; Peres 2000Peres CA. 2000. Identifying keystone plant resources in tropical forests: the case of gums from Parkia pods. Journal of Tropical Ecology 16: 287-317.; Gomes et al. 2010Gomes VSM, Buckeridge MS, Silva CO, Scarano FR, Araujo DSD, Alves MAS. 2010. Availability peak of caloric fruits coincides with energy-demanding seasons for resident and non-breeding birds in restinga, an ecosystem related to the Atlantic forest, Brazil. Flora 205: 647-655.; Sebastian-González 2017Sebastián‐González E. 2017. Drivers of species' role in avian seed‐dispersal mutualistic networks. Journal of Animal Ecology 86: 878-887.); the latter often being constrained by abiotic factors (Fenner 1998Fenner M. 1998. The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, Evolution and Systematics 1: 78-91.; Mendoza et al. 2017Mendoza I, Peres CA, Morellato LPC. 2017. Continental-scale patterns and climatic drivers of fruiting phenology: A quantitative Neotropical review. Global and Planetary Change 148: 227-241.). Besides the temporal variation, fruit availability also shows considerable heterogeneity in space (Howe 1984Howe HF. 1984. Implications of seed dispersal by animals for tropical reserve management. Biological Conservation 30: 261-281.; Levey 1988Levey DJ. 1988. Spatial and temporal variation in Costa Rican fruit and fruit-eating bird abundance. Ecological Monographs 58: 251-269.; García & Ortiz-Pulido 2004García D, Ortiz-Pulido R. 2004. Patterns of resource tracking by avian frugivores at multiple spatial scales: two case studies on discordance among scales. Ecography 27: 187-196.; Price 2004Price OF. 2004. Indirect evidence that frugivorous birds track fluctuating fruit resources among rainforest patches in the Northern Territory, Australia. Austral Ecology 29: 137-144.). In this context, birds are known to track seasonal changes in resource availability across distinct habitats in patchy landscapes (Price 2004Price OF. 2004. Indirect evidence that frugivorous birds track fluctuating fruit resources among rainforest patches in the Northern Territory, Australia. Austral Ecology 29: 137-144.; Tubelis et al. 2004Tubelis DP, Cowling A, Donnelly C. 2004. Landscape supplementation in adjacent savannas and its implications for the design of corridors for forest birds in the central Cerrado, Brazil. Biological Conservation 118: 353-364.; Piratelli & Blake 2006Piratelli A, Blake JG. 2006. Bird communities of the southeastern Cerrado region, Brazil. Ornitologia Neotropical 17: 213-225.; Maruyama et al. 2013Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99-109.). Furthermore, the morphological and nutritional characteristics of fruits are known to determine the associations between plants and frugivores (Stiles 1993Stiles EW. 1993. The influence of pulp lipids on fruit preference by birds. Vegetatio 107/108: 227-235.; Galetti et al. 2011Galetti M, Pizo MA, Morellato LPC. 2011. Diversity of functional traits of fleshy fruits in a species-rich Atlantic rain forest. Biota Neotropica 11: 181-193.; Jordano 2014Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.), and fruit energetic content dictates how important specific fruit bearing plants are for frugivores (Sebastian-González 2017Sebastián‐González E. 2017. Drivers of species' role in avian seed‐dispersal mutualistic networks. Journal of Animal Ecology 86: 878-887.).
Despite the accumulated knowledge about how plant-frugivore interactions are structured and their importance for community dynamics (Jordano 2014Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.), the actual resource availability considering nutritional characteristics of fruits, according to distinct habitats and seasonality, has been insufficiently characterized in most biodiverse regions of the world (Mendoza et al. 2017Mendoza I, Peres CA, Morellato LPC. 2017. Continental-scale patterns and climatic drivers of fruiting phenology: A quantitative Neotropical review. Global and Planetary Change 148: 227-241.). This is especially important in the tropics, where birds more specialized on fruit diet comprise higher proportions of frugivores when compared to temperate areas (Dalsgaard et al. 2017Dalsgaard B, Schleuning M, Maruyama PK, et al. 2017. Opposed latitudinal patterns of network‐derived and dietary specialization in avian plant-frugivore interaction systems. Ecography 40: 1395-1401.). The Cerrado of Central Brazil is the most biodiverse savanna ecosystem in the world, characterized both by strong seasonality and diversity of distinct habitats that form a landscape mosaic (Silva & Bates 2002Silva JMC, Bates JM. 2002. Biogeographic patterns and conservation in the South American Cerrado: A tropical savanna hotspot. BioScience 52: 225-234.). A recent survey has shown that out of the ca. 12,000 vascular plant species in the Cerrado (Mendonça et al. 2008Mendonça RC, Felfili JM, Walter BMT, et al. 2008. Flora vascular do bioma Cerrado: checklist com 12.356 espécies. In: Sano SM, Almeida SP, Ribeiro JF. (eds.) Cerrado: Ecologia e Flora. Vol. 2. Brasília, Embrapa Cerrados/Embrapa Informação Tecnológica. p. 421-1279.; BFG 2015BFG-The Brazil Flora Group. 2015. Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.), ca. 4,000 species of plants are associated with animals for seed dispersal (Kuhlmann & Ribeiro 2016b Kuhlmann M, Ribeiro JF. 2016b. Evolution of seed dispersal in the Cerrado biome: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 271-282.). Moreover, many of the plant and animal species participating in these frugivory interactions are found in more than one type of habitat (grassland, savanna and forest) that characterize the Cerrado (Kuhlmann & Ribeiro 2016aKuhlmann M, Ribeiro JF. 2016a. Fruits and frugivores of the Brazilian Cerrado: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 495-507.; bKuhlmann M, Ribeiro JF. 2016b. Evolution of seed dispersal in the Cerrado biome: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 271-282.).
This species overlap illustrates a complex dynamic of Cerrado at the landscape scale. For instance, while zoochorous fruit availability is markedly seasonal in more open habitats of the Cerrado (Piratelli & Pereira 2002Piratelli A, Pereira MR. 2002. Dieta de aves na região leste de Mato Grosso do Sul, Brasil. Ararajuba 10: 131-139.; Batalha & Martins 2004Batalha MA, Martins FR. 2004. Reproductive phenology of the cerrado plant community in Emas National Park (central Brazil). Australian Journal of Botany 52: 149-161.; Oliveira 2008Oliveira PE. 2008. Fenologia e biologia reprodutiva das espécies de Cerrado. In: Sano SM, Almeida SP, Ribeiro JF. (eds.) Cerrado: Ecologia e Flora . Vol. 1. Brasília, Embrapa Cerrados/Embrapa Informação Tecnológica . p. 273-290.), it can be less seasonal in more forested areas where there is a more constant supply of fleshy fruits (Melo et al. 2013Melo C, Silva AM, Oliveira PE. 2013. Oferta de frutos por espécies zoocóricas de sub-bosque em Gradiente florestal do Cerrado. Bioscience Journal 29: 2030-2041.). Even though birds frequently track resources connecting distinct habitats in this environment, including open savannas, palm swamps and forests (Tubelis et al. 2004Tubelis DP, Cowling A, Donnelly C. 2004. Landscape supplementation in adjacent savannas and its implications for the design of corridors for forest birds in the central Cerrado, Brazil. Biological Conservation 118: 353-364.; Piratelli & Blake 2006Piratelli A, Blake JG. 2006. Bird communities of the southeastern Cerrado region, Brazil. Ornitologia Neotropical 17: 213-225.; Maruyama et al. 2013Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99-109.), simultaneous evaluations of distinct habitats are lacking in the literature. Such connections among habitats have important consequences for conservation of the Cerrado (Tubelis et al. 2004Tubelis DP, Cowling A, Donnelly C. 2004. Landscape supplementation in adjacent savannas and its implications for the design of corridors for forest birds in the central Cerrado, Brazil. Biological Conservation 118: 353-364.; Kuhlmann & Ribeiro 2016aKuhlmann M, Ribeiro JF. 2016a. Fruits and frugivores of the Brazilian Cerrado: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 495-507.), also in the context of plants and frugivorous birds interactions (e.g. Maruyama et al. 2013Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99-109.; Purificação et al. 2014Purificação KN, Pascotto MC, Pedroni F, Pereira JMN, Lima NA. 2014. Interactions between frugivorous birds and plants in savanna and forest formations of the Cerrado. Biota Neotropica 14: e20140068. doi: 10.1590/1676-06032014006814
https://doi.org/10.1590/1676-06032014006...
). Moreover, identifying plants that provide fruits during periods of scarcity could help identify potential “keystone resources” (Peres 2000Peres CA. 2000. Identifying keystone plant resources in tropical forests: the case of gums from Parkia pods. Journal of Tropical Ecology 16: 287-317.; Bleher et al. 2003Bleher B, Potgieter CJ, Johnson DN, Böhning-Gaese K. 2003. The importance of figs for frugivores in a South African coastal forest. Journal of Tropical Ecology 19: 375-386.; Maruyama et al. 2013Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99-109.), which should be considered in conservation and restoration planning.
Seed dispersal by birds is the most common strategy for zoochorous Cerrado plants, present in almost 60 % of the species and genera, and 80 % of zoochorous plant families (Kuhlmann & Ribeiro 2016aKuhlmann M, Ribeiro JF. 2016a. Fruits and frugivores of the Brazilian Cerrado: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 495-507.). In this study, we evaluated the fruiting phenology of the most abundant plant species associated with frugivorous birds in distinct habitat types for three years, and recorded fruit morphology and nutritional characteristics. Moreover, based on previous studies conducted in the same region, we assessed the overlap in interactions with frugivorous birds among a subset of the studied plants. Our major goal is to highlight how distinct habitats in the Cerrado are interconnected by the interactions between plants and animals, especially regarding frugivory.
Materials and methods
Study sites and phenological sampling
Data collection was carried out from May 2008 to May 2011 in three distinct areas around the city of Uberlândia, state of Minas Gerais, Brazil. Sampling was conducted at the (1) “Clube de Caça e Pesca Itororó de Uberlândia”, a private natural reserve (hereafter CCPIU - 18°59'21"S 48°18'06"W) comprised of approximately 400 ha of native vegetation, with two major plant physiognomies: savanna and palm swamp, the latter comprised of about 100 ha; (2), Panga Ecological Station (Panga - 19°10'27"S 48°23'51"W), which has about 400 ha and includes open grasslands and savanna to dense forest formations, such as gallery forests (Cardoso et al. 2009Cardoso E, Moreno MIC, Bruna EM, Vasconcelos HL. 2009. Mudanças fitofisionômicas no Cerrado: 18 anos de sucessão ecológica na Estação Ecológica do Panga, Uberlândia-MG. Caminhos de Geografia 10: 254-268.); (3) forest remnant within the “Parque Municipal do Sabiá (Sabiá - 18°54'36"S 48°13'46"W), an urban park with 185 ha, of which 35 ha are occupied by natural vegetation, mostly semi-deciduous forest and palm swamp, subjected to human disturbance (Amorim & Oliveira 2006Amorim FW, Oliveira PE. 2006. Estrutura sexual e ecologia reprodutiva de Amaioua guianensis Aubl. (Rubiaceae), uma espécie dióica de formações florestais de cerrado. Revista Brasileira de Botânica 29: 353-362.). We chose these three remnant areas of natural vegetation to obtain a sufficient representation of the remarkable physiognomic variation that characterizes the Cerrado - Neotropical Savanna of Central Brazil (Oliveira-Filho & Ratter 2002Oliveira-Filho AT, Ratter JÁ. 2002. Vegetation physiognomies and woody flora of the cerrado biome. In: Oliveira PS, Marquis RJ. (eds.). The cerrados of Brazil: Ecology and natural history of a Neotropical savanna. New York, Columbia University Press. p. 91-120.). The climate in the region is considered Cwa according to Köppen classification, with a warm rainy season (October - March) and a cool dry season (April - September) combined with strong seasonality (Fig. 1; Cardoso et al. 2009Cardoso E, Moreno MIC, Bruna EM, Vasconcelos HL. 2009. Mudanças fitofisionômicas no Cerrado: 18 anos de sucessão ecológica na Estação Ecológica do Panga, Uberlândia-MG. Caminhos de Geografia 10: 254-268.; Alvares et al. 2013Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G. 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, 22: 711-728.). The mean annual temperature is 20.9 oC and the annual precipitation in the region is 1,524 mm (Alvares et al. 2013Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G. 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, 22: 711-728.).
Walter and Lieth climate diagram for Uberlândia, Minas Gerais, using the historical series data (> 25 years) from Alvares et al. (2013Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G. 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, 22: 711-728.). The temperature curve is shown in red, and the precipitation bars are shown in blue/yellow. When the precipitation bars are above the temperature curve (blue bars), this period is relatively humid. On the other hand, when the precipitation bars are below the temperature curve (yellow bars), the period is relatively dry. When the precipitation bars are above 100 mm, this period is relatively rainy.
Each study area was visited fortnightly for three years of data collection, totaling 69 records in the field. During each of these visits, we walked along transects running through the different vegetation types in each area, covering a distance of about 4.0 km in CCPIU (3.0 km of typical savanna and 1.0 km of palm swamp with patches of forest), 4.0 km in Panga (2.0 km of savanna and 2.0 km of forest, the latter including both gallery and semi-deciduous forests) and 1.5 km in Sabiá (semi-deciduous forest), according to the size of the natural vegetation area and representativeness of distinct plant physiognomies. All transects had a width of 5 m. When walking in the transects, we recorded the phenology of bird consumed fruits. Initially, we chose plant species based on previous knowledge about bird-fruit interactions in the region (see below), and we restricted our study to plant species that were represented by at least 10 distinct fruiting individuals in the transects of the different study areas. These species were marked and followed throughout the sampling period. We did this because we were interested in the most abundant resources for birds and our nutritional profile analysis required considerable amounts of fruit pulp to be sampled (see below). Species that did not have 10 fruiting individuals or did not produce enough fruits for nutritional analysis were subsequently excluded from sampling. The only exception for this threshold was Copaifera langsdorffii (Fabaceae), as we only found five reproductive individuals in one year, which were large trees with ample fruit production. This gave us a total of 35 plant species, from 15 families (Fig. 2, Tabs. 1, 2). Rubiaceae (seven spp.), Melastomataceae (six spp.) and Myrtaceae (five spp.) were the most diverse plant families sampled. Regarding habitats, 57.1 % (20 spp.) were sampled in open savanna, 34.3 % (12 spp.) in the forest and 2.8 % (one sp.) in the palm swamp, while Cecropia pachystachya (Urticaceae) was found in the latter two habitats. Species names and families were checked on the Brazilian Flora webpage (http://floradobrasil.jbrj.gov.br). Individuals of the abundant plants were surveyed, and fruiting phenology was evaluated by assigning intensity scores: 0 - no fruits; 1 - less than 40 % of individuals with fruits; 2 - more than 40 % of individuals with fruits (see Maruyama et al. 2013Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99-109.). This is an adaptation of the activity index (Bencke & Morellato 2002Bencke CS, Morellato LPC. 2002. Comparação de dois métodos de avaliação da fenologia de plantas, sua interpretação e representação. Brazilian Journal of Botany 25: 269-275.), which considers the presence and absence of a specific phenological phase in each plant individual. We only considered ripe fruits in our sampling. Voucher specimens from the studied populations can be found in the Herbarium Uberlandense (HUFU, Tab. S1 in supplementary material).
Fruits consumed by birds in the Cerrado of Central Brazil. A. Hirtella glandulosa, B. Rourea induta, C. Davilla elliptica, D. Doliocarpus dentatus, E. Erythroxylum deciduum, F. Copaifera langsdorffii, G. Lacistema hasslerianum, H. Byrsonima intermedia, I. Miconia albicans, J. Miconia ibaguensis, K. Tococa guianensis, L. Eugenia calycina, M. Eugenia ligustrina, N. Myrcia guianensis, O. Ouratea hexasperma, P. Coussarea hydrangeifolia, Q. Faramea hyacinthina, R. Palicourea rigida, S. Psychotria carthagenensis, T. Psychotria platypoda, U. Rudgea viburnoides, V. Smilax brasiliensis, W. Smilax quinquenervia, X. Styrax ferrugineus, Y. Cecropia pachystachya.
Morphological parameters of the most abundant ornithochorous fruits in the Cerrado of Uberlândia, state of Minas Gerais, Brazil. For all species, we measured the diaspores, except for C. pachystachya, in which we measured infructescences. For this species and Melastomataceae, we did not precisely measure the seeds, as these were all very small.
Nutritional profile of the most abundant ornithochorous fruits in the Cerrado of Uberlândia, state of Minas Gerais, Brazil. All values are in percentages, and for nutritional values other than water, values refer to the dry mass.
Morphological and nutritional traits of the fruits
For each plant species, we collected ripe fruits for morphological and nutritional content analysis. Only freshly picked fruits were used for morphological measurements, while for nutritional analysis fruits were frozen and stored until enough material was accumulated (no longer than six months). For the morphological analysis, we used the dispersal unit, i.e., diaspore, as the sampling unit. This could be the whole fruit, as for drupes and berries, seed with arils, or part of the fruit containing seeds, as in infructescences or compound fruits (Kuhlmann & Ribeiro 2016aKuhlmann M, Ribeiro JF. 2016a. Fruits and frugivores of the Brazilian Cerrado: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 495-507.). For simplicity, we henceforth refer to all of these as fruits. Sampling was always spread out among distinct individuals by sampling more than 10 individuals for the morphological analysis and characterization of the nutritional profile. For each fruit/diaspore we measured: 1) fruit length, taken longitudinally in relation to the peduncle; 2) fruit width, taken as the largest length perpendicular to fruit length; 3) fruit weight; 4) fruit dry weight, recorded after fresh fruits were left in a dry chamber at 70 ⁰C for five days; 5) number of seeds per fruit; 6) seed length, considering the largest length of each individual seed; 7) seed width, the largest perpendicular length of the seed, in relation to (6); and 8) seed weight, adding the total weight of all seeds in the fruit. For each measurement, we always tried to get a sample size of 100. In C. pachystachya, the delimitation of “diaspore” was difficult, since the nutritional tissue is the enlarged infructescence axis and the perianth remnants surrounding the seed, making it hard to estimate the exact amount removed by the animals at each visit (Lobova et al. 2003Lobova TA, Mori SA, Blanchard F, Peckham H, Charles‐Dominique P. 2003. Cecropia as a food resource for bats in French Guiana and the significance of fruit structure in seed dispersal and longevity. American Journal of Botany 90: 388-403.). Thus, for this species we report the size of the infructescence, and not of the diaspore, which were usually fragments of the elongated sorose snatched by frugivores. In this case, to estimate water content and seed/fruit weight ratio, we cut 1 cm cylindrical pieces from the infructescence, which were used for subsequent measurements.
For each species, we separated the pulp or aril from the seeds, except for Melastomataceae species and C. pachystachya, which we tried to separate from the tiny seeds (ca. 1mm), however, some seeds remained within the samples. The high variability in fruit size and pulp content resulted in a highly variable number of fruits sampled for each species, but we aimed to sample at least 40 g of pulp for sugar, protein, lipid and ash (minerals) content analyses. All samples were kept frozen until the time of analysis. For sugar content analysis, we used High-performance liquid chromatography (HPLC), which identified the types of sugar present in the samples and their proportions (Burgner & Feinberg 1992Burgner E, Feinberg M. 1992. Determination of mono - and disaccharides in foods by interlaboratory study: Quantification of bias components for liquid chromatography. Journal of AOAC International 75: 443-464.). For lipid content, we used the Soxhlet extraction method (Zenebon et al. 2008Zenebon O, Pascuet NS, Tiglea P. 2008. Métodos físico-químicos para análise de alimentos. 1st eletronic edn. São Paulo, Instituto Adolfo Lutz); for proteins we used the Kjeldahl method, which quantifies the nitrogen content and then multiplies it by a fixed value to estimate the protein content (LANARA 1981LANARA - Laboratório Nacional de Referência Animal. 1981. Métodos analíticos oficiais para controle de produtos de origem animal e seus ingredientes. II- Métodos físicos e químicos. Brasília, Ministério da Agricultura.), using a conversion factor of 5.64, which is appropriate for fruit pulp (Levey et al. 2000Levey DJ, Bissell HA, O'keefe SF. 2000. Conversion of nitrogen to protein and amino acids in wild fruits. Journal of Chemical Ecology 26: 1749-1763.). Finally, the residual ash after incineration at 550-570 ⁰C was used to estimate the mineral content (Zenebon et al. 2008Zenebon O, Pascuet NS, Tiglea P. 2008. Métodos físico-químicos para análise de alimentos. 1st eletronic edn. São Paulo, Instituto Adolfo Lutz).
Frugivorous birds
We surveyed previous studies conducted in the study region that assessed the assemblage of frugivorous birds associated with the plants recorded in our study (Melo et al. 2003Melo C, Bento EC, Oliveira PE. 2003. Frugivory and dispersal of Faramea cyanea (Rubiaceae) in cerrado wood plant formations. Brazilian Journal of Biology 63: 75-82.; Melo & Oliveira 2009Melo C, Oliveira PE. 2009. Frugivory in Lacistema hasslerianum Chodat (Lacistemaceae), a gallery forest understory treelet in Central Brazil. Brazilian Journal of Biology 69: 631-637. ; Maruyama et al. 2013Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99-109.; Silva & Melo 2013Silva AM, Melo C. 2013. Overlap and resource sharing in coteries of fruit-eating birds. Journal of Tropical Ecology 29: 409-416.; Silva & Pedroni 2014Silva GBM, Pedroni F. 2014. Frugivory by birds in cerrado in the city of Uberlândia, Minas Gerais. Revista Árvore 38: 433-442.; Silva et al. 2016Silva AM, Maruyama PK, Paniago LPM, Melo C. 2016. Modularity in ecological networks between frugivorous birds and congeneric plant species. Journal of Tropical Ecology 32: 526-535.). From these studies, we extracted information about the bird species recorded consuming the fruits of each plant species. Since these studies were based on distinct sampling design and effort, we only used the presence/absence of interactions between birds and plants to generate a matrix of plants × birds interactions. We checked for additional studies in the Google Scholar® database, using plant species names as keywords, but no additional studies were found for the region. We were able to compile a dataset on frugivore birds associated with 16 of the 35 studied plant species, from 10 distinct plant families (Tab. S2 in supplementary material), which was used in the subsequent analysis.
Analyses
We tested if fruiting phenology was evenly distributed throughout the year in the forest and open savanna Cerrado formations, considering the presence/absence of ripe fruits. The palm swamp was not included in the analysis as only one exclusive species, Miconia chamissois (Melastomataceae), was sampled in this formation. However, including this species as an open savanna species in the analysis did not change the results (not shown). As we surveyed the plant community fortnightly, the circumference representing the year was divided by 24, and the midpoint between two sectors represented the months. For each year, we calculated the circular standard deviation, circular mean (μ), and the length of the mean vector (r), which represents how the data is clustered around the mean (0 - perfectly uniformly distributed, 1 - perfectly clustered). We then performed single sample distribution Rayleigh's tests for each year, where p < 0.05 indicates unimodal distribution and, therefore, seasonality in fruiting patterns (Morellato et al. 1989Morellato LPC, Rodrigues RR, Leitão Filho HF, Joly CA. 1989. Estudo comparativo da fenologia de espécies arbóreas de floresta de altitude e floresta mesófila semi-decídua na Serra do Japi, Jundiaí, São Paulo. Revista Brasileira de Botânica 12: 85-98.; 2010Morellato LPC, Alberti LF, Hudson IL. 2010. Applications of circular statistics in plant phenology: a case studies approach. In: Hudson IL, Keatley MR. (eds.) Phenological research. Dordrecht, Springer. p. 339-359.). Circular analyses were performed in the circular package (Lund et al. 2017Lund U, Agostinelli C, Arai H, et al. 2017. circular: Circular Statistics. R Package Version 0.4-93. https://CRAN.R-project.org/package=circular
https://CRAN.R-project.org/package=circu...
). Since we performed multiple tests for each year/environment, we applied a Bonferroni correction of p-values to avoid type I error.
We explored the morphological and nutritional data of the fruits with non-Metric Multidimensional Scaling (nMDS) analysis to see how plants are distributed in the ordination considering the entire assemblage. We performed nMDS ordinations separately for morphological and nutritional data. For morphological data, as different units of measurements were used, we first standardized data to zero mean and unit variance, then computed Euclidian distances among species according to their morphological data using the vegan package (Oksanen et al. 2018Oksanen AJ, Blanchet FG, Friendly M, et al. 2018. Vegan: Community Ecology Package. R Packag. version 2.5-1. https://CRAN.R-project.org/package=vegan
https://CRAN.R-project.org/package=vegan...
). We removed C. pachystachya from this analysis, since we were unable to delimit individual diaspores. Moreover, we combined the fruit/seed length and width into a single measurement called “size”, calculated as the sum of length and width divided by 2, to reduce the number of variables. For nutritional variables, we also standardized data to zero mean and unit variance and computed Euclidian distances among species. The resulting dissimilarity matrices were used for the two distinct nMDS plots.
Finally, we calculated the Sørensen dissimilarity index between plant species based on presence/absence data of the birds associated with fruits. The resulting dissimilarity matrix was used for a hierarchical clustering using the UPGMA agglomeration method. This analysis was conducted with the vegan package (Oksanen et al. 2018Oksanen AJ, Blanchet FG, Friendly M, et al. 2018. Vegan: Community Ecology Package. R Packag. version 2.5-1. https://CRAN.R-project.org/package=vegan
https://CRAN.R-project.org/package=vegan...
). All analyses were conducted in R (R Development Core Team 2016R Development Core Team. 2016. R: A language and environment for statistical computing. Vienna, The R Foundation. https://www.r-project.org/
https://www.r-project.org/...
).
Results
Of the 35 plant species, most (62.8 %, 22 spp.) fruited during the rainy season, while 28.6% (10 spp.) fruited during the dry season and three had no clear associations to seasons (Fig. 3). Interestingly, when contrasting the phenology in relation to habitats, 85.0% of the plants from open savanna fruited during the rainy season, while in the forest habitat only a slight majority, 53.8%, fruited during the dry season (Figs. 3, 4). Of the two species found in the palm swamp, Miconia chamissois fruited during the dry season, while C. pachystachya showed no clear fruiting seasonality (the latter was also found in forest areas). Considering the seven plant families with more than one sampled species, different species from the same families often occur in both open savanna and forest habits. The number of plants that produced ripe fruits in open savanna had a unimodal distribution throughout the three years, peaking during the rainy season (mean vector directions were comprised between December and January; Fig. 4). On the other hand, fruiting seasonality was not found in forest areas (Fig. 4).
Fruiting phenology of the most abundant plants associated with frugivorous birds in Uberlândia, state of Minas Gerais, Brazil. The phenology was evaluated fortnightly (69 sampling events) by attributing intensity scores: 0 - no fruits (white); 1 - less than 40% of individuals with fruits (grey); 2 - more than 40% of individuals with fruits (black). The two seasons of Cerrado (rainy - October to March and dry - April to September) are separated by vertical lines. Species are listed according to their habitat and fruiting phenology. Filled and open circles indicate the most sugar rich and lipid rich species, respectively.
Rose diagrams showing the number of species producing ripe fruits (in green) in the savanna and forest formations for three years. Letters around the circle indicate the months (clockwise) and numbers inside the circles indicate the mean number of species with fruits in the respective month. Vectors indicating the length and direction of the mean (in red) are shown only for the significant results. Blue and beige shades indicate relatively humid and relatively dry periods, respectively (according to Fig. 1).
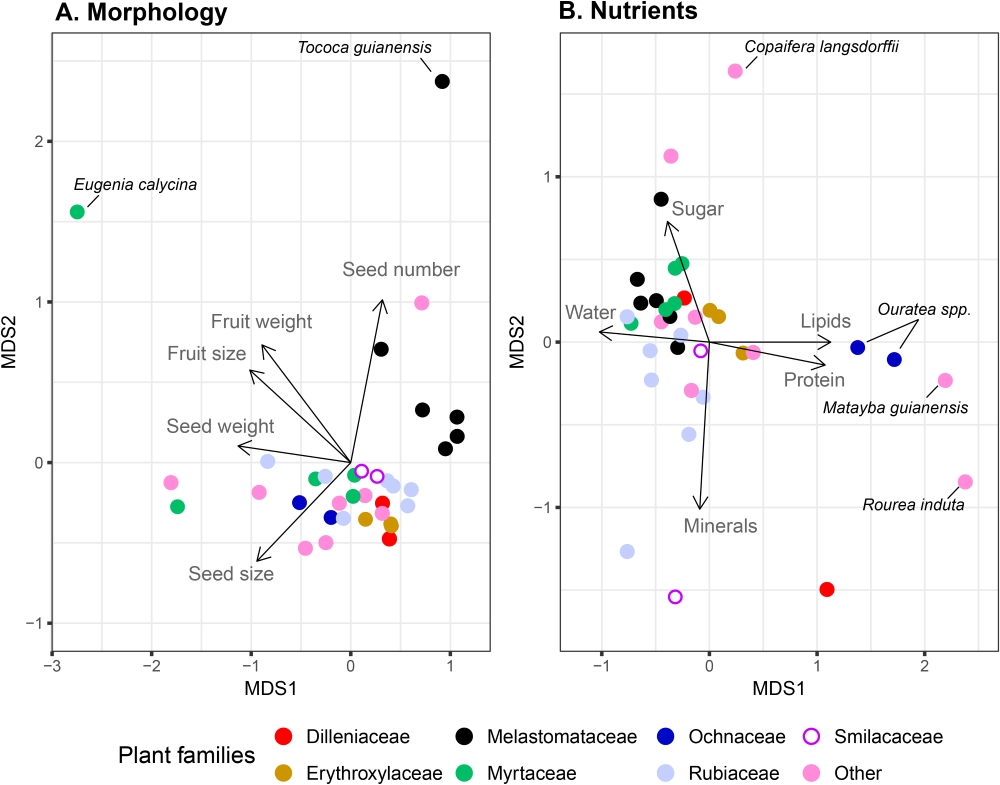
Fruits/diaspores (excluding C. pachystachya) showed average dimensions of 8.5 ± 2.9 mm length and 7.3 ± 1.9 mm width (Tab. 1). Seed number per fruit varied greatly across species, from 1 in several species to more than 100 seeds in Tococa guianensis (Melastomataceae). Likewise, seed/fruit ratio in weight also varied greatly from 9.1 % in Miconia fallax (Melastomataceae) to 91.7 % in Matayba guianensis (Sapindaceae, Tab. 1). The nMDS with morphological data resulted in a two-dimension solution with stress = 3.9 % (R2 = 0.99; Fig. 5 A ). When considering the nutritional profile, the nMDS with nutritional data resulted in a two-dimensional solution with stress = 9.9 % (R2 = 0.99; Fig. 5 B ). All plant species had either sugar or lipids as the major nutrient in the fruits, with the majority, 77.1 % (27 spp.), providing more sugar than lipids. Two types of sugars were found in fruits, fructose and glucose, although no differences in the proportion of these sugars were found across plant species (t-test, paired for each species, t = 1.55, df = 34, p = 0.13). Species such as Rudgea viburnoides (Rubiaceae), Miconia rubiginosa (Melastomataceae), Styrax ferrugineus (Styracaceae) and Copaifera langsdorffii had the highest proportion of sugar in their fruits (> 17.0 %), with the latter having as much as ca. 25 % of its dry mass composed of sugar. In contrast, most lipid rich species had very low amounts of sugar (≤ 1.0 %), with species such as Rourea induta (Connaraceae), Matayba guianensis, Ouratea hexasperma and O. spectabilis (Ochnaceae) producing fruits with more than 25.0 % of lipid content (Tab. 2). All species providing moderate (> 7.0 %) to high lipid content fruits produced fruits during the rainy season and were found only in the open savanna habitat (Tab. 2, Figs. 3, 5B). Visually, for both morphological and nutritional parameters, species from the same families tended to be grouped in the nMDS ordinations (Fig. 5 A, B ).
non-Metric Multidimensional Scaling (nMDS) ordination of the morphological (A) and nutritional (B) characteristics of the Cerrado plants. The seven families with most species are represented, while all the families not discriminated in the plots are included in “Other”. Plants with the most distinct characteristics from the assemblage are indicated in the plots.
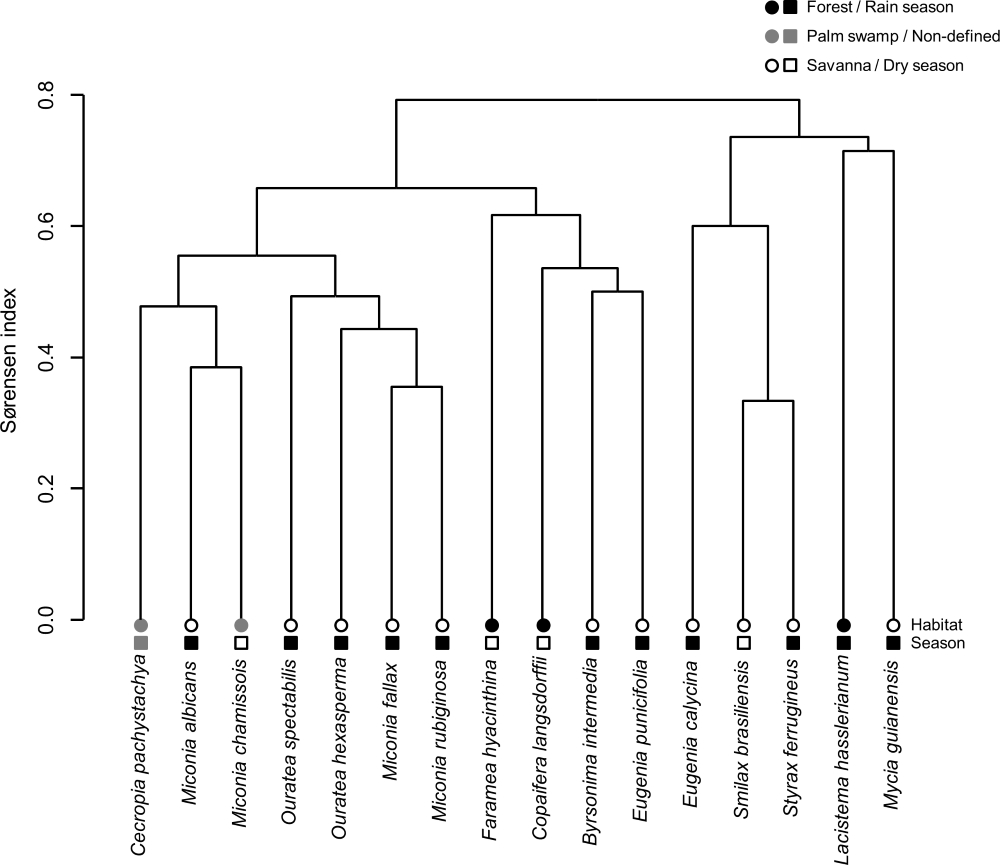
We found 74 frugivorous bird species associated with the 16 plant species included in our survey (Tab. S2 in supplementary material). When considering the bird assemblages associated with the plants, there was no clear clustering of plant species according to habitat or fruiting season, indicating that plants from savanna, palm swamp and forest habitats often share frugivore bird species throughout the seasons (Fig. 6).
Hierarchical clustering of plant species according to their interactions with frugivore birds in the Cerrado of Uberlândia, Brazil. We used the UPGMA agglomeration method based on Sørensen dissimilarity index considering presence/absence data of each pairwise bird-plant interaction. Plants were classified according to their main habitat (circles; forest, palm swap, savanna) and fruiting season (squares; rainy season, dry season or non-defined).
Discussion
Our results about seasonality combined with nutritional profile stress the importance of distinct habitats in providing fruits for frugivorous birds at different times of the year and with distinct nutritional characteristics in the Cerrado. Although community-wide studies about fruit availability and nutritional traits have previously been conducted in some species rich tropical ecosystems (e.g., Wheelwright et al. 1984Wheelwright NT, Haber WA, Murray KG, Guindon C. 1984. Tropical fruit-eating birds and their food plants: a survey of a Costa Rican lower montane forest. Biotropica 16: 173-192.; Gomes et al. 2010Gomes VSM, Buckeridge MS, Silva CO, Scarano FR, Araujo DSD, Alves MAS. 2010. Availability peak of caloric fruits coincides with energy-demanding seasons for resident and non-breeding birds in restinga, an ecosystem related to the Atlantic forest, Brazil. Flora 205: 647-655.; Galetti et al. 2011Galetti M, Pizo MA, Morellato LPC. 2011. Diversity of functional traits of fleshy fruits in a species-rich Atlantic rain forest. Biota Neotropica 11: 181-193.), they did not emphasize different contiguous habitats and complementarity. The difference in fruiting peak patterns among habitats, with seasonality in the open savanna habitat and the absence of seasonality in the forest, conforms with previous studies conducted in the Cerrado (Piratelli & Pereira 2002Piratelli A, Pereira MR. 2002. Dieta de aves na região leste de Mato Grosso do Sul, Brasil. Ararajuba 10: 131-139.; Batalha & Martins 2004Batalha MA, Martins FR. 2004. Reproductive phenology of the cerrado plant community in Emas National Park (central Brazil). Australian Journal of Botany 52: 149-161.; Oliveira 2008Oliveira PE. 2008. Fenologia e biologia reprodutiva das espécies de Cerrado. In: Sano SM, Almeida SP, Ribeiro JF. (eds.) Cerrado: Ecologia e Flora . Vol. 1. Brasília, Embrapa Cerrados/Embrapa Informação Tecnológica . p. 273-290.; Melo et al. 2013Melo C, Silva AM, Oliveira PE. 2013. Oferta de frutos por espécies zoocóricas de sub-bosque em Gradiente florestal do Cerrado. Bioscience Journal 29: 2030-2041.; Brito et al. 2017Brito VLG, Maia FR, Silveira FAO, et al. 2017. Reproductive phenology of Melastomataceae species with contrasting reproductive systems: contemporary and historical drivers. Plant Biology 19: 806-817.). It has been suggested that distribution of distinct habitats in the Cerrado is associated with soil characteristics, including humidity, which may in turn affect habitat specific phenology (Furley 1999Furley PA. 1999. The nature and diversity of Neotropical savanna vegetation with particular reference to the Brazilian cerrados. Global Ecology and Biogeography 8: 223-241.). Although the pattern of fruiting peak during the rainy season has previously been reported in the Cerrado woodland (Mendoza et al. 2017Mendoza I, Peres CA, Morellato LPC. 2017. Continental-scale patterns and climatic drivers of fruiting phenology: A quantitative Neotropical review. Global and Planetary Change 148: 227-241.), our study adds to previous knowledge by evaluating distinct habitats simultaneously, as well as by combining phenology with the morphological and nutritional characteristics of the fruits.
In general, the 35 studied plant species had small fruits/diaspores, characteristic of bird consumed fruits (Jordano 2014Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.). Accordingly, previous findings suggest that ca. 80 % of zoochorous fruits in Cerrado are up to 10 mm (Kuhlmann & Ribeiro 2016aKuhlmann M, Ribeiro JF. 2016a. Fruits and frugivores of the Brazilian Cerrado: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 495-507.). One correlation that is often observed in different assemblages of zoochorous fruits is that carbohydrate/sugar rich and watery pulp fruits contain a large number of smaller seeds, while lipid rich fruits have low carbohydrates with larger seeds surrounded by pulp (Wheelwright et al. 1984Wheelwright NT, Haber WA, Murray KG, Guindon C. 1984. Tropical fruit-eating birds and their food plants: a survey of a Costa Rican lower montane forest. Biotropica 16: 173-192.; Galetti et al. 2011Galetti M, Pizo MA, Morellato LPC. 2011. Diversity of functional traits of fleshy fruits in a species-rich Atlantic rain forest. Biota Neotropica 11: 181-193.; Jordano 2014Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.). In our analysis of the Cerrado, species from the most diverse plant families (Rubiaceae, Melastomataceae and Myrtaceae) had watery fruits with sugar as the major nutritional component. Fruits of Melastomataceae plants also contained many tiny seeds. Numerous tiny seeds associated with sugar-rich pulp were also observed in C. pachystachya, whose fruits are consumed both by bats (Sato et al. 2008Sato TM, Passos FDC, Nogueira AC. 2008. Frugivoria de morcegos (Mammalia, Chiroptera) em Cecropia pachystachya (Urticaceae) e seus efeitos na germinação das sementes. Papéis Avulsos de Zoologia 48: 19-26.) and birds (Silva & Pedroni 2014Silva GBM, Pedroni F. 2014. Frugivory by birds in cerrado in the city of Uberlândia, Minas Gerais. Revista Árvore 38: 433-442.; Purificação et al. 2014Purificação KN, Pascotto MC, Pedroni F, Pereira JMN, Lima NA. 2014. Interactions between frugivorous birds and plants in savanna and forest formations of the Cerrado. Biota Neotropica 14: e20140068. doi: 10.1590/1676-06032014006814
https://doi.org/10.1590/1676-06032014006...
), indicating generalist seed dispersal interactions. None of these plants had significant lipid content, which is a characteristic often associated with fruits consumed by more specialized frugivores (Stiles 1993Stiles EW. 1993. The influence of pulp lipids on fruit preference by birds. Vegetatio 107/108: 227-235.; Jordano 2014Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.). All lipid rich fruits/diaspores of Rourea induta (Connaraceae), Ouratea spp. (Ochnaceae) and Matayba guianensis (Sapindaceae) had a relatively large single seed. It should be noted that these correlations are not rules (Jordano 2014Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.), as illustrated by Copaifera langsdorffii, which has the most sugar rich aril associated to relatively large seeds. Finally, morphological and nutritional characteristics of fruits are constrained by evolutionary relationships (Jordano 1995Jordano P. 1995. Angiosperm fleshy fruits and seed dispersers: a comparative analysis of adaptation and constraints in plant-animal interactions. The American Naturalist 145: 163-191.). In line with this, our ordination analyses also suggested the importance of plant phylogeny, as species of the same families tended to be clumped together in the morphological ordination space and had the same major nutritional rewards (Fig. 5).
Differences in lipid/sugar nutritional content of fruits also respond to climate, since lipids are metabolically more expensive for plants to produce and are more commonly found in fruits produced during favorable seasons (Jordano 2014Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.). In turn, fruiting phenology of plants has potentially greater effects on frugivores than vice versa (Schaik et al. 1993Schaik CP, Terborgh JW, Wright SJ. 1993. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics 24: 353-377.; Fenner 1998Fenner M. 1998. The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, Evolution and Systematics 1: 78-91.), such that the annual cycle of reproduction, breeding and migratory movements of these animals are often correlated with fruiting seasonality (Gomes et al. 2010Gomes VSM, Buckeridge MS, Silva CO, Scarano FR, Araujo DSD, Alves MAS. 2010. Availability peak of caloric fruits coincides with energy-demanding seasons for resident and non-breeding birds in restinga, an ecosystem related to the Atlantic forest, Brazil. Flora 205: 647-655.; Jordano 2014Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.). Community wide studies on reproduction of Cerrado birds have shown that most species, including frugivores, show reproductive peak during the rainy season (Marini & Durães 2001Marini MÂ, Durães R. 2001. Annual patterns of molt and reproductive activity of passerines in south-central Brazil. The Condor 103: 767-775.; Marini et al. 2012Marini MÂ, Borges FJ, Lopes LE, et al. 2012. Breeding biology of birds in the Cerrado of central Brazil. Ornitologia Neotropical 23: 385-405.). The lipid rich species producing fruits during the rainy season in the savanna habitat may be important resources for these birds during this critical time in their life cycles. Moreover, fruiting seasonality in these formations could attract even migratory species, as lipid rich fruits have been linked to migratory patterns (Herrera 1995Herrera CM. 1995. Plant-vertebrate seed dispersal systems in the Mediterranean: ecological, evolutionary, and historical determinants. Annual Review of Ecology and Systematics 26: 705-727.; Gomes et al. 2010Gomes VSM, Buckeridge MS, Silva CO, Scarano FR, Araujo DSD, Alves MAS. 2010. Availability peak of caloric fruits coincides with energy-demanding seasons for resident and non-breeding birds in restinga, an ecosystem related to the Atlantic forest, Brazil. Flora 205: 647-655.).
The considerable overlap in interactions of birds and plants from distinct habitats and fruiting seasons highlights the link between habitats at the landscape level. This supports previous studies showing that vegetation characteristics that delimit the different grassland, savanna and forest habitats of the Cerrado do not prevent birds from tracking resources in space (Tubelis et al. 2004Tubelis DP, Cowling A, Donnelly C. 2004. Landscape supplementation in adjacent savannas and its implications for the design of corridors for forest birds in the central Cerrado, Brazil. Biological Conservation 118: 353-364.; Piratelli & Blake 2006Piratelli A, Blake JG. 2006. Bird communities of the southeastern Cerrado region, Brazil. Ornitologia Neotropical 17: 213-225.; Maruyama et al. 2013Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99-109.). In this sense, although there are differences in the composition of fruit eating birds in forest and savanna habitats (Purificação et al. 2014Purificação KN, Pascotto MC, Pedroni F, Pereira JMN, Lima NA. 2014. Interactions between frugivorous birds and plants in savanna and forest formations of the Cerrado. Biota Neotropica 14: e20140068. doi: 10.1590/1676-06032014006814
https://doi.org/10.1590/1676-06032014006...
), around 60 % of frugivorous animals in the Cerrado occur in more than one type of habitat (Kuhlmann & Ribeiro 2016aKuhlmann M, Ribeiro JF. 2016a. Fruits and frugivores of the Brazilian Cerrado: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 495-507.). Other studies have also indicated how interactions between plants and frugivorous birds connect and are maintained across distinct habitats (e.g. Maruyama et al. 2013Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99-109.; Purificação et al. 2014Purificação KN, Pascotto MC, Pedroni F, Pereira JMN, Lima NA. 2014. Interactions between frugivorous birds and plants in savanna and forest formations of the Cerrado. Biota Neotropica 14: e20140068. doi: 10.1590/1676-06032014006814
https://doi.org/10.1590/1676-06032014006...
). Hence, distinct Cerrado vegetation types are interdependent, which is a key feature that should be considered in conservation and restoration policies for this ecosystem.
In summary, temporal availability of bird consumed fruits varies across distinct habitats in the Cerrado. We are aware that not all frugivorous birds are effective seed dispersers, but the latter are a subset of the former. Therefore, the results obtained have implications not only for conservation of frugivorous birds, but also for seed dispersal interactions. Throughout the Cerrado, the number and proportion of zoochorous plant species is higher in the forest than in open habitats (Kuhlmann & Ribeiro 2016aKuhlmann M, Ribeiro JF. 2016a. Fruits and frugivores of the Brazilian Cerrado: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 495-507.; bKuhlmann M, Ribeiro JF. 2016b. Evolution of seed dispersal in the Cerrado biome: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 271-282.). Hence, the higher number of ornithochorous plant species found in the savanna is likely a consequence of our sampling that only focused on the most common/abundant species and included 5.0 km transects in savanna vs. 3.5 km in forest. Nevertheless, our results stress that there are also many abundant and highly rewarding plants with bird-consumed fruits in open Cerrado areas. By showing differences in the temporal availability of fruits between habitats and in the nutritional quality of the pulp, we call attention to the complementarity between habitats for maintaining plant-frugivore interactions and the potential for seed dispersal, an essential process for regeneration of plant communities. We hope that our study contributes to more integrated conservation planning for the Cerrado and other ecosystems with landscape mosaics.
Acknowledgements
FAPEMIG and CNPq (ref. 431873/2018-6) provided essential funding. We thank Ivan Schiavini, Glein M. Araújo and Rosana Romero for help with plant identification, Adriano Silva for help with bird survey and two anonymous reviewers for suggestions that improved the quality of the manuscript. Pietro K. Maruyama is also very grateful to his parents Isumi and Dario, his sister Mayara and his partner Amanda for valuable support in processing thousands of fruits for subsequent analyses (apologies for the stained fingers!).
References
- Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G. 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, 22: 711-728.
- Amorim FW, Oliveira PE. 2006. Estrutura sexual e ecologia reprodutiva de Amaioua guianensis Aubl. (Rubiaceae), uma espécie dióica de formações florestais de cerrado. Revista Brasileira de Botânica 29: 353-362.
- Batalha MA, Martins FR. 2004. Reproductive phenology of the cerrado plant community in Emas National Park (central Brazil). Australian Journal of Botany 52: 149-161.
- Bencke CS, Morellato LPC. 2002. Comparação de dois métodos de avaliação da fenologia de plantas, sua interpretação e representação. Brazilian Journal of Botany 25: 269-275.
- BFG-The Brazil Flora Group. 2015. Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.
- Bleher B, Potgieter CJ, Johnson DN, Böhning-Gaese K. 2003. The importance of figs for frugivores in a South African coastal forest. Journal of Tropical Ecology 19: 375-386.
- Brito VLG, Maia FR, Silveira FAO, et al 2017. Reproductive phenology of Melastomataceae species with contrasting reproductive systems: contemporary and historical drivers. Plant Biology 19: 806-817.
- Burgner E, Feinberg M. 1992. Determination of mono - and disaccharides in foods by interlaboratory study: Quantification of bias components for liquid chromatography. Journal of AOAC International 75: 443-464.
- Cardoso E, Moreno MIC, Bruna EM, Vasconcelos HL. 2009. Mudanças fitofisionômicas no Cerrado: 18 anos de sucessão ecológica na Estação Ecológica do Panga, Uberlândia-MG. Caminhos de Geografia 10: 254-268.
- Dalsgaard B, Schleuning M, Maruyama PK, et al 2017. Opposed latitudinal patterns of network‐derived and dietary specialization in avian plant-frugivore interaction systems. Ecography 40: 1395-1401.
- Fenner M. 1998. The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, Evolution and Systematics 1: 78-91.
- Fleming TH, Kress WJ. 2011. A brief history of fruits and frugivores. Acta Oecologica 37: 521-530.
- Furley PA. 1999. The nature and diversity of Neotropical savanna vegetation with particular reference to the Brazilian cerrados. Global Ecology and Biogeography 8: 223-241.
- Galetti M, Pizo MA, Morellato LPC. 2011. Diversity of functional traits of fleshy fruits in a species-rich Atlantic rain forest. Biota Neotropica 11: 181-193.
- García D, Ortiz-Pulido R. 2004. Patterns of resource tracking by avian frugivores at multiple spatial scales: two case studies on discordance among scales. Ecography 27: 187-196.
- Gomes VSM, Buckeridge MS, Silva CO, Scarano FR, Araujo DSD, Alves MAS. 2010. Availability peak of caloric fruits coincides with energy-demanding seasons for resident and non-breeding birds in restinga, an ecosystem related to the Atlantic forest, Brazil. Flora 205: 647-655.
- Herrera CM. 1995. Plant-vertebrate seed dispersal systems in the Mediterranean: ecological, evolutionary, and historical determinants. Annual Review of Ecology and Systematics 26: 705-727.
- Howe HF. 1984. Implications of seed dispersal by animals for tropical reserve management. Biological Conservation 30: 261-281.
- Jordano P. 1995. Angiosperm fleshy fruits and seed dispersers: a comparative analysis of adaptation and constraints in plant-animal interactions. The American Naturalist 145: 163-191.
- Jordano P. 2014. Fruits and frugivory. In: Gallagher RS. (ed.) Seeds: the ecology of regeneration in plant communities. Wallingford, CAB International. p. 18-61.
- Kuhlmann M, Ribeiro JF. 2016a. Fruits and frugivores of the Brazilian Cerrado: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 495-507.
- Kuhlmann M, Ribeiro JF. 2016b. Evolution of seed dispersal in the Cerrado biome: ecological and phylogenetic considerations. Acta Botanica Brasilica 30: 271-282.
- LANARA - Laboratório Nacional de Referência Animal. 1981. Métodos analíticos oficiais para controle de produtos de origem animal e seus ingredientes. II- Métodos físicos e químicos. Brasília, Ministério da Agricultura.
- Levey DJ. 1988. Spatial and temporal variation in Costa Rican fruit and fruit-eating bird abundance. Ecological Monographs 58: 251-269.
- Levey DJ, Bissell HA, O'keefe SF. 2000. Conversion of nitrogen to protein and amino acids in wild fruits. Journal of Chemical Ecology 26: 1749-1763.
- Lobova TA, Mori SA, Blanchard F, Peckham H, Charles‐Dominique P. 2003. Cecropia as a food resource for bats in French Guiana and the significance of fruit structure in seed dispersal and longevity. American Journal of Botany 90: 388-403.
- Lund U, Agostinelli C, Arai H, et al 2017. circular: Circular Statistics. R Package Version 0.4-93. https://CRAN.R-project.org/package=circular
» https://CRAN.R-project.org/package=circular - Marini MÂ, Borges FJ, Lopes LE, et al 2012. Breeding biology of birds in the Cerrado of central Brazil. Ornitologia Neotropical 23: 385-405.
- Marini MÂ, Durães R. 2001. Annual patterns of molt and reproductive activity of passerines in south-central Brazil. The Condor 103: 767-775.
- Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99-109.
- Melo C, Bento EC, Oliveira PE. 2003. Frugivory and dispersal of Faramea cyanea (Rubiaceae) in cerrado wood plant formations. Brazilian Journal of Biology 63: 75-82.
- Melo C, Oliveira PE. 2009. Frugivory in Lacistema hasslerianum Chodat (Lacistemaceae), a gallery forest understory treelet in Central Brazil. Brazilian Journal of Biology 69: 631-637.
- Melo C, Silva AM, Oliveira PE. 2013. Oferta de frutos por espécies zoocóricas de sub-bosque em Gradiente florestal do Cerrado. Bioscience Journal 29: 2030-2041.
- Mendonça RC, Felfili JM, Walter BMT, et al 2008. Flora vascular do bioma Cerrado: checklist com 12.356 espécies. In: Sano SM, Almeida SP, Ribeiro JF. (eds.) Cerrado: Ecologia e Flora. Vol. 2. Brasília, Embrapa Cerrados/Embrapa Informação Tecnológica. p. 421-1279.
- Mendoza I, Peres CA, Morellato LPC. 2017. Continental-scale patterns and climatic drivers of fruiting phenology: A quantitative Neotropical review. Global and Planetary Change 148: 227-241.
- Morellato LPC, Alberti LF, Hudson IL. 2010. Applications of circular statistics in plant phenology: a case studies approach. In: Hudson IL, Keatley MR. (eds.) Phenological research. Dordrecht, Springer. p. 339-359.
- Morellato LPC, Rodrigues RR, Leitão Filho HF, Joly CA. 1989. Estudo comparativo da fenologia de espécies arbóreas de floresta de altitude e floresta mesófila semi-decídua na Serra do Japi, Jundiaí, São Paulo. Revista Brasileira de Botânica 12: 85-98.
- Oksanen AJ, Blanchet FG, Friendly M, et al 2018. Vegan: Community Ecology Package. R Packag. version 2.5-1. https://CRAN.R-project.org/package=vegan
» https://CRAN.R-project.org/package=vegan - Oliveira PE. 2008. Fenologia e biologia reprodutiva das espécies de Cerrado. In: Sano SM, Almeida SP, Ribeiro JF. (eds.) Cerrado: Ecologia e Flora . Vol. 1. Brasília, Embrapa Cerrados/Embrapa Informação Tecnológica . p. 273-290.
- Oliveira-Filho AT, Ratter JÁ. 2002. Vegetation physiognomies and woody flora of the cerrado biome. In: Oliveira PS, Marquis RJ. (eds.). The cerrados of Brazil: Ecology and natural history of a Neotropical savanna. New York, Columbia University Press. p. 91-120.
- Peres CA. 2000. Identifying keystone plant resources in tropical forests: the case of gums from Parkia pods. Journal of Tropical Ecology 16: 287-317.
- Piratelli A, Blake JG. 2006. Bird communities of the southeastern Cerrado region, Brazil. Ornitologia Neotropical 17: 213-225.
- Piratelli A, Pereira MR. 2002. Dieta de aves na região leste de Mato Grosso do Sul, Brasil. Ararajuba 10: 131-139.
- Price OF. 2004. Indirect evidence that frugivorous birds track fluctuating fruit resources among rainforest patches in the Northern Territory, Australia. Austral Ecology 29: 137-144.
- Purificação KN, Pascotto MC, Pedroni F, Pereira JMN, Lima NA. 2014. Interactions between frugivorous birds and plants in savanna and forest formations of the Cerrado. Biota Neotropica 14: e20140068. doi: 10.1590/1676-06032014006814
» https://doi.org/10.1590/1676-06032014006814 - R Development Core Team. 2016. R: A language and environment for statistical computing. Vienna, The R Foundation. https://www.r-project.org/
» https://www.r-project.org/ - Sato TM, Passos FDC, Nogueira AC. 2008. Frugivoria de morcegos (Mammalia, Chiroptera) em Cecropia pachystachya (Urticaceae) e seus efeitos na germinação das sementes. Papéis Avulsos de Zoologia 48: 19-26.
- Schaik CP, Terborgh JW, Wright SJ. 1993. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics 24: 353-377.
- Sebastián‐González E. 2017. Drivers of species' role in avian seed‐dispersal mutualistic networks. Journal of Animal Ecology 86: 878-887.
- Silva AM, Melo C. 2013. Overlap and resource sharing in coteries of fruit-eating birds. Journal of Tropical Ecology 29: 409-416.
- Silva AM, Maruyama PK, Paniago LPM, Melo C. 2016. Modularity in ecological networks between frugivorous birds and congeneric plant species. Journal of Tropical Ecology 32: 526-535.
- Silva GBM, Pedroni F. 2014. Frugivory by birds in cerrado in the city of Uberlândia, Minas Gerais. Revista Árvore 38: 433-442.
- Silva JMC, Bates JM. 2002. Biogeographic patterns and conservation in the South American Cerrado: A tropical savanna hotspot. BioScience 52: 225-234.
- Stiles EW. 1993. The influence of pulp lipids on fruit preference by birds. Vegetatio 107/108: 227-235.
- Tubelis DP, Cowling A, Donnelly C. 2004. Landscape supplementation in adjacent savannas and its implications for the design of corridors for forest birds in the central Cerrado, Brazil. Biological Conservation 118: 353-364.
- Wheelwright NT, Haber WA, Murray KG, Guindon C. 1984. Tropical fruit-eating birds and their food plants: a survey of a Costa Rican lower montane forest. Biotropica 16: 173-192.
- Zenebon O, Pascuet NS, Tiglea P. 2008. Métodos físico-químicos para análise de alimentos. 1st eletronic edn. São Paulo, Instituto Adolfo Lutz
Publication Dates
-
Publication in this collection
12 Sept 2019 -
Date of issue
Jul-Sep 2019
History
-
Received
26 June 2019 -
Accepted
27 July 2019