Abstracts
Cytokeratins (CK) belong to the intermediate filament group and are expressed in epithelial cells. Their expression is tissue specific and for this reason they are the most important markers of epithelial differentiation. Monoclonal antibodies, which specifically mark one CK, are used in the diagnosis of many tumors. In the last decade, several mutations were described in CK genes, which lead to structural changes in its molecule. As a result, the pathogenesis of many skin diseases has been clarified, such as Epidermolysis bullosa simplex (CK 5 or 14), Epidermolytic hyperkeratosis (CK 1 or 10), Epidermolytic palmoplantar keratoderma (CK 9) and Pachyonychia congenita (CK 6, 16 or 17).
cytokeratin; immunohistochemistry; molecular genetics; mutation
As citoqueratinas (CQ) são constituintes do citoesqueleto das células epiteliais, pertencendo aos filamentos intermediários; sua distribuição é específica para cada subtipo de epitélio, permitindo que sejam utilizadas como importantes marcadores de sua diferenciação. Anticorpos monoclonais permitem sua localização nos tecidos e são utilizados no diagnóstico de tumores. Na última década inúmeras mutações foram descritas em seus genes, levando a alteração em sua estrutura molecular, esclarecendo várias enfermidades cutâneas, como epidermólise bolhosa simples (CQ 5 ou 14), hiperqueratose epidermolítica (CQ 1 ou 10), hiperqueratose palmoplantar epidermolítica (CQ 9) e paquioníquia congênita (CQ 6, 16 ou 17).
citoqueratinas; imuno-histoquímica; genética molecular; mutação
CONTINUING MEDICAL EDUCATION
Cytokeratins* * Work done at "Universidade Federal e Católica de Pelotas".
Hiram Larangeira de Almeida Jr.
Adjunct Professor of Dermatology, Universidade Federal de Pelotas and of the Masters degree course in Health and Behaviour, Universidade Católica de Pelotas
Correspondence Correspondence to Prof. Dr. Hiram Larangeira de Almeida Jr. Universidade Católica de Pelotas Mestrado em Saúde e Comportamento Rua Almirante Barroso,1202 - 107 - Bloco G 96010-280 Pelotas RS E-mail: hiramalmeidajr@hotmail.com
SUMMARY
Cytokeratins (CK) belong to the intermediate filament group and are expressed in epithelial cells. Their expression is tissue specific and for this reason they are the most important markers of epithelial differentiation. Monoclonal antibodies, which specifically mark one CK, are used in the diagnosis of many tumors. In the last decade, several mutations were described in CK genes, which lead to structural changes in its molecule. As a result, the pathogenesis of many skin diseases has been clarified, such as Epidermolysis bullosa simplex (CK 5 or 14), Epidermolytic hyperkeratosis (CK 1 or 10), Epidermolytic palmoplantar keratoderma (CK 9) and Pachyonychia congenita (CK 6, 16 or 17).
Key-words: cytokeratin; immunohistochemistry; molecular genetics; mutation;
Cytokeratins (CK) are components of the cytoskeleton of epithelial cells and as a consequence are also denominated epithelial keratin or soft keratin, and should be differentiated from the trichokeratins, or the so-called hard keratin, which form the hair shaft and nails.1
The cytoskeleton is an intracellular proteic network, constituted by intermediate filaments, which measure from seven to 10 nm in thickness; by actin filaments, of about 7 nm; and by the microtubules, that measure 25 nm.2 The microtubules are related to the intracellular transport of organelles, the actin filaments participate in the cellular mobility, and intermediate filaments provide the three-dimensional cell structure.2
Three subclasses of intermediate filaments have been defined: vimentin and related (vimentin present in the mesenchymal cells, desmins in the myocytes and glial proteins in the neuroglial cells); neurofilaments (present in the neurons); and finally the cytokeratins, found in the epithelia and structures derived from them.2
The intermediate filaments are capable of autopolymerization, forming a cytoplasmatic network responsible for the mechanical strength of the cells, and in the case of the epithelia, are important against eventual simple trauma.3,4
CK have been classified through two-dimensional electrophoresis,5 which separates the proteins not only by their molecular weight, but also on the account of pH. Using this technique it was observed that the pattern obtained in the electrophoresis varied, for instance, if it was performed using epidermal or follicular lysate (Figure 1). They are currently divided into two groups: type I that are acid and include CK from 9 to 23; and type II that are alkaline, comprising CK from 1 to 8.4
The CK constitute the largest group of intermediate filaments, with over 20 different types described to date, and they are of extreme importance for understanding various mechanisms in cutaneous diseases.
Likewise, the trichokeratins are also divided into two groups, denominated according to convention as group I from hHa1 to hHa8 (human Hair acidic) and group II from hHb1 to hHb6 (human Hair basic), according to their location in the two-dimensional electrophoresis.6
More recently, four different variants of CK 6 have been described in the inner root sheath (IRS)and denominated K6irs1, K6irs2, K6irs3 and K6irs4;7,8 other variants, denominated 6a and 6b, have a different distribution in the cutaneous annexes, and are responsible for the clinical variability of certain diseases (see mutations).9
Their importance in the pathogenesis of several dermatoses is due to the specificity of the expression of CK according to the epithelium involved, for instance, the epidermis or the corneal epithelium, and also because they can be expressed only in certain sectors of the epithelia.
Most of the time, they are found in pairs (Table 1), forming heterodymers, or in other words, the union of two different CK, constituting filaments, which are the three-dimensional structure forming the cytoskeleton, and are anchored in the desmosomes and in the internal plaque of the hemidesmosomes.
SPECIFIC TISSULAR DISTRIBUTION
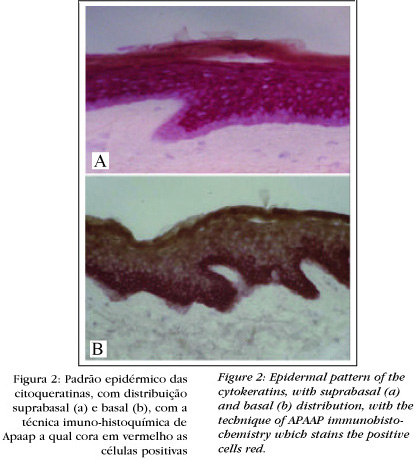
CK have a characteristic distribution in each epithelium and its annexes. In the epidermis, for instance, they can have a basal expression (Figure 2) - CK 5 and 14 - or suprabasal - CK 1 and 10. In the hair follicle basal CK and hyperproliferative CK are found - CK 6, 16 and 17 - they received this denomination as they are also found in pathological situations, such as in the epidermis of psoriasis and in tumors. CK 19 is found in the outermost layer of the outer root ar sheath, also denominated the basal layer of this sheath, this being more specific to the hair follicle (Figure 3). The follicular section above the sebaceous gland expresses the same CK as the epidermis.
Other epithelia, such as the simple, corneal and the stratified non-keratinizing also have their form of CK (Table 1).
In this manner, it is possible to consider follicular and epidermal patterns of distribution for the CK. These specific patterns allow CK to be used as important markers for epithelial differentiation.
As mentioned above, CK 6 and CK 16 are expressed in the epidermis in situations where hyperproliferation occurs, such as in psoriasis and in epithelial tumors, for which it has also been denominated hyperproliferative CK. The experimental induction of CK 6 has already been demonstrated, when the epidermis is stimulated by cytokines, such as interferon gamma, epithelial growth factor (EGF) and tumor necrosis factor (TNF), as well as by ultraviolet radiation, and the latter also induces CK 19, thus illustrating the dynamic aspects of CK expression.10,11
All the CK have a similar molecule, constituted by four helical segments - 1A, 1B, 2A and 2B - interspersed by short non-helical segments, called ligand segments L1, L12 and L2. In the extremity the variable segments V1 and V2 are found, while basic CK present segments H1 and H2 between the helical and variable segments4,9,12 (Figure 4). Despite such a close similarity, monoclonal antibodies have been developed that are capable of marking each CK.
The use of these monoclonal antibodies allows, for instance, the determination of which CK are found in a given tumor and the origin of these cells, based on molecular markers and not just their morphologic aspects.
USE IN IMMUNOHISTOCHEMISTRY
The application of monoclonal antibodies markers of CK enables an investigation into the origin of tumors and their differential diagnosis.
Basal cell carcinomas express basal CK 5 and 14, CK 17 and CK 19 (Figure 5), a pattern similar to that of the hair follicle,13,14 and the possibility has been suggested that they originate from the outer root sheath,15 which correlates with clinical findings, since this tumor is not found in palmar or plantar areas.
Likewise, the identification of CK 20 is already used in the diagnosis of Merkel cell carcinoma,16 as well as in the precocious identification of metastases in the sentinel lymph nodes.17
Also in Mohs' micrographic surgery, the demarcation with antibodies against CK enables an increase in the success rate for this type of surgical treatment,18 because cells that are difficult to visualize with hematoxylin and eosin can be identified with the antibodies.
Antibodies with low specificity that mark several CK, can be used in the diagnosis of undifferentiated neoplasias; since when expressing these epithelial markers, it can be affirmed that it is an undifferentiated carcinoma, thereby differentiating it from the lymphomas, for example.
In a similar way, antivimentin antibodies are used in the diagnosis of mesenchymal tumors, however the great number of cytokeratins means that their use in the diagnosis of the epithelial neoplasias is much more widespread than in the case of the vimentin.
MUTATIONS
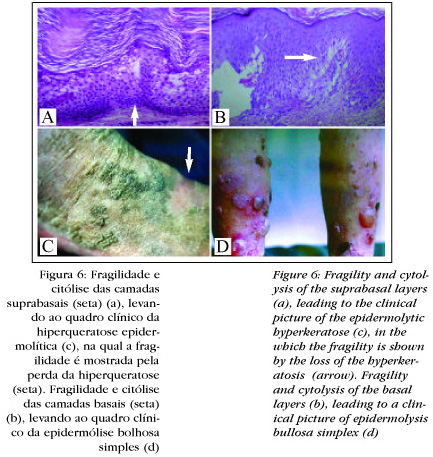
Another important application for this new knowledge is in the genodermatoses due to mutation of CKs. In that as they are expressed in a localized form, disease consequent to this mutation will be specific to certain cells and certain epithelial segments. They are generally associated with cytolysis, since the cell collapses as a result of alterations in the cytoskeleton.
The alteration of the basal layer by mutation, whether CK 5 or 14, leads to the degeneration of the basal layer (Figure 6), with the fragility and formation of blisters, characteristic of epidermolysis bullosa simplex (EBS)19,20 the first illness in which the mutation of a CK.21 was demonstrated.22 In the disseminated forms of the disease the mutations are located in the extremities of the helical domain, or in other words, at the beginning of 1A or at the end of 2B;23 in the palmoplantar forms they are located in the region of L1221 (Figure 4).
It has already been demonstrated in vitro that the mutation of CK 14 impedes normal polymerization, besides presenting less resistance and elasticity than the normal heteropolymer.3 Another alteration has indicated that keratinocytes of patients with EBS are more sensitive to osmotic shock in vitro and take longer to recover than normal cells,22 thereby demonstrating the decrease in their resistance, that culminates in vivo in blisters.
In a similar manner to the fragility and degeneration of the basal layer, as seen in EBS, alterations of the suprabasal CK lead to a degeneration in the upper epidermis, characteristic of epidermolytic hyperkeratosis (EH) (Figure 6). Also in this illness mutations have been described in CK, namely CK 1 or CK 10, that are expressed in the same layers of the epidermis.24-26 Patients with a CK 10 mutation tend to present a more severe clinical picture, while a CK 1 mutation is accompanied by palmoplantar hyperkeratosis. In patients with a defect in CK 10, it is possible that CK 9, present in the stratum lucidum, compensates for the defect in CK 10, since they belong to the same group of acid CKs,26 mutations of CK 10 have already been described with palmar and plantar involvement.27
Mutations of CK 9, since it is found in the palmoplantar epidermis, are accompanied by a degeneration restricted to that area, typical of epidermolytic palmoplantar keratoderma;28-30 likewise, in this disease the mutations have been found at the beginning of segment 1 A.30
According to the same principle between the specific location of the normal expression of a CK and that of the illness due to its mutations, one can also explain the clinical variability of congenital pachyonychia. In type 1, characterized by ungual alteration plus palmoplantar hyperkeratosis and oral leukoderma, mutations have been detected in the CK 6a and 16 found in these regions.31,32 In type 2, besides the thickening of the ungual blade, cysts can occur at puberty, that are often difficult to differentiate from the subtypes in infancy.31 In this variant, mutations have been described in CK 1733,34 and later in a family case in CK 6b.
In steatocystoma multiplex, whose cysts are similar to those of type 2 pachyonychia congenita, mutations have also been described in CK 17 - including identical mutations, that can be considered clinical variants, since in some cases there is also mild ungual involvement,31 other genetic factors must be involved, as this would explaining the different phenotypes resulting from the same mutations,31 however, these have yet to be clarified.
In recent years, it has been possible to explain the molecular genetics of some corneal dystrophies with mutations of CK present in that epithelium. In corneal Meesman dystrophy, mutations have been described in CK 3 and 12. Clinically opacity and vesicles intraepithelial are seen, and any of two CK can be involved, leading to the same ophthalmologic clinical picture, in a way very similar to that which occurs in EBS.35-37
In the simple epithelium, found in the liver, pancreas and intestine, illnesses have also been verified associated to mutations, involving the cytoskeletons, described recently. In some patients with cryptogenic cirrhosis, which occurs without viral hepatitis, alcoholism or any other known cause, mutations have been described in CK 8 and 18.38-39
New information in this area has contributed to further correlations between phenotype and genotype, and has allowed a better understanding of the pathogenesis and clinical variability of many dermatoses;40,41 Knowledge of these mutations can also be used in the prenatal diagnosis based on DNA obtained by chorionic biopsy,42,43 which can be performed around week 10 of gestation, thereby substituting skin biopsy by fetoscopy done between the week 18 and 20. Complications in the latter occur between 4 and 7% of the cases, as opposed to just 1% in chorionic biopsy.
The information obtained in the last two decades on CK has brought countless advances in the understanding of various cutaneous diseases, thereby demonstrating the importance of laboratorial research and its subsequent application in daily practice. q
REFERENCES
Received in December, 11rd of 2003
Approved by the Editorial Council and accepted for publication in January 27th of 2004
Questions and Answers to Questions
1. No citoesqueleto das células epiteliais não encontramos:
a. Queratinas duras.
b. Microtúbulos.
c. Citoqueratinas.
d. Filamentos de actina.
2. Não é função do citoesqueleto:
a. Participar da motilidade celular.
b. Auxiliar no transporte de organelas.
c. Produzir a haste do cabelo.
d. Dar a estrutura tridimensional da célula.
3. Qual das proteínas abaixo não é um filamento intermediário?
a. Vimentina.
b. Laminina.
c. Desmina.
d. Neurofilamentos.
4. Assinale a alternativa incorreta:
a. As citoqueratinas são constituídas por quatro seg mentos helicoidais (1A, 1B, 2A, 2B).
b. A homologia molecular entre as diversas citoquerati nas impede o desenvolvimento de anticorpos mono clonais.
c. Os segmentos helicoidais são intercalados por seg mentos de ligação (L1, L12 e L2).
d. As citoqueratinas básicas têm também os segmentos H1 e H2.
5. Qual afirmação abaixo é incorreta?
a. As citoqueratinas têm distribuição tecidual específica.
b. As citoqueratinas formam em muitos epitélios hete rodímeros.
c. As citoqueratinas ancoram-se nos desmossomas e hemidesmossomas.
d. O padrão de citoqueratinas de um epitélio não sofre variações.
6. Assinale a afirmativa incorreta:
a. As citoqueratinas são divididas em ácidas e básicas.
b. Na bainha radicular interna encontram-se variantes da CQ 6.
c. As citoqueratinas não são capazes de se autopolimerizar.
d.As tricoqueratinas são divididas em ácidas e básicas.
7. Assinale a afirmativa incorreta:
a. A epiderme expressa citoqueratinas diferentes na camada basal e nas camadas suprabasais.
b. O folículo piloso expressa as citoqueratinas basais.
c. Na psoríase não ocorre mudança nas citoqueratinas epidérmicas.
d. O setor folicular acima da glândula sebácea tem um padrão epidérmico de citoqueratinas.
8. Com relação ao carcinoma basocelular, assinale a afirmativa correta:
a. São encontradas as citoqueratinas basais e supraba- sais.
b. São encontradas as citoqueratinas suprabasais.
c. O padrão encontrado é o da bainha radicular interna.
d. O padrão encontrado é o da bainha radicular externa.
9. Em qual das situações abaixo a demarcação imuno-histoquímica das citoqueratinas é negativa?
a. Nos carcinomas indiferenciados.
b. No carcinoma de Merkel.
c. No carcinoma basocelular.
d. No dermatofibrossarcoma protuberante.
10. Em qual das formas abaixo de epidermólise bolhosa foram descritas mutações em citoqueratinas?
a. Epidermólise bolhosa simples
b. Epidermólise bolhosa juncional
c. Epidermólise bolhosa distrófica
d. Epidermólise bolhosa adquirida
11. Assinale a afirmativa incorreta.
a. Na psoríase encontram-se na epiderme as chamadas citoqueratinas hiperproliferativas.
b. A célula de Merkel expressa a CQ 20.
c. A CQ 19 é encontrada em todas as células tumorais do carcinoma basocelular.
d. A bainha radicular externa expressa as chamadas citoqueratinas hiperproliferativas.
12. Em quais citoqueratinas ainda não foi descrita mutação?
a. CQ 1 e 10.
b. CQ 5 e 14.
c. CQ 3 e 12.
d. CQ 19 e 20.
13. A alteração celular mais vista na doenças por mutação das citoqueratinas é:
a. Cariorrexe.
b. Citólise.
c. Espongiose.
d. Acantólise.
14. Em que parte do gen das citoqueratinas basais localiza-se a maioria das mutações da epidermólise bolhosa simples palmoplantar?
a. No início do segmento 1A.
b. No final do segmento 2B.
c. No segmento não-helicoidal L12.
d. No início do segmento 2B.
15. Em qual doença existe variabilidade clínica, dependendo da CQ mutada?
a. Epidermólise bolhosa simples
b. Cirrose criptogênica
c. Paquioníquia congênita
d. Distrofia corneana
16. Na cirrose criptogênica foram descritas mutações nas CQ:
a. CQ 8 e 18.
b. CQ 6 e 16.
c. CQ 1 e 11.
d. CQ 9 e 19.
17. Na paquiníquia congênita foram descritas mutações nas CQ:
a. CQ 6.
b. CQ 16.
c. CQ 17.
d. Todas as anteriores.
18. Na hiperqueratose epidermolítica foram descritas mutações nas CQ:
a. CQ 8 e 18.
b. CQ 6 e 16.
c. CQ 1 e 10.
d. CQ 5 e 14.
19. Assinale a alternativa incorreta:
a. As informações sobre mutações podem ser utilizadas no diagnóstico pré-natal a partir de biópsia coriônica.
b. A alteração histológica da distrofia corneana de Meesmann é semelhante à da epidermólise bolhosa simples.
c. Na queratodermia palmoplantar epidermolítica foram descritas mutações na CQ 9.
d. Até o momento não é possível fazer correlações genofenotípicas.
20. Na distrofia corneana de Meesmann foram descritas mutações nas CQ:
a. CQ 8 e 18.
b. CQ 3 e 12.
c. CQ 1 e 10.
d. CQ 5 e 14.
GABARITO
Malformações Vasculares
2004; 79(1): 07-25
1. b 9. c
2. c 10. a
3. d 11. a
4. c 12. d
5. a 13. c
6. d 14. b
7. b 15. d
8. b
- 1. Langbein L, Rogers MA, Winter H et al The catalog of human hair keratins I. Expression of the nine type I members in the hair follicle. J Biol Chem 1999; 274: 19.874-84.
- 2. Alberts B, Bray D, Johnson A et al O Citoesqueleto. In: Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Fundamentos da Biologia Celular- Uma introdução à biologia molecular da célula. Porto Alegre: Artmed 1999: 526-60.
- 3. Ma L, Xu J, Coulombe PA, Wirtz D. Keratin filament suspension show unique micromechanical properties. J Biol Chem 1999; 274: 19.145- 51.
- 4. Coulombe PA, Omary MB. 'Hard' and 'soft' principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol 2002; 14: 110-22.
- 5. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982; 31:11-24.
- 6. Langbein L, Rogers MA, Winter H, Praetzel S, Schweizer J. The catalog of human hair keratins II.Expression of the six type II members in the hair follicle and the combined catalog of human type I and II keratins. J Biol Chem 2001; 276: 35123-32.
- 7. Langbein L, Rogers MA, Praetzel S, Winter H, Schweizer J. K6irs1, K6irs2, K6irs3 and K6irs4 represent the inner-root-sheath-specific type II epithelial keratins of the human hair follicle. J Invest Dermatol 2003; 120: 512-22.
- 8. Langbein L, Rogers MA, Praetzel S, Aoki N, Winter H, Schweizer J. A novel epithelial keratin, hK6irs1, is expressed differentially in all layers of the inner root sheath, including specialized Huxley cells (Flügelzellen) of the human hair follicle. J Invest Dermatol 2001; 118: 789-99.
- 9. Smith FJD. The molecular genetics of keratin disorders. Am J Clin Dermatol 2003; 4: 347-364.
- 10. Bernerd F, Del Bino S, Asselineau. Regulation of keratin expression by ultraviolet radiation: differential and specific effects of ultraviolet B and ultraviolet A exposure. J Invest Dermatol 2001; 117: 1421-9.
- 11. Hattori N, Komine M, Yano et al Interferon-?, a strong supressor of cell proliferation, induces upregulation of K6, one of the inflammatory- and proliferation-associated keratins. J Invest Dermatol 2002; 119: 403-10.
- 12. Ishida-Yamamoto A, Takahashi H, Lizuka H. Lessons from disorders of epidermal differentiation-associated keratins. Histol Histopathol 2002; 17: 331-8.
- 13. Schirren CG, Rutten A, Kaudewitz P, Diaz C, McClain S, Burgdorf WH. Trichoblastoma and basal cell carcinoma are neoplasms with follicular differentiation sharing the same profile of cytokeratin intermediate filaments. Am J Dermatopathol 1997; 19: 341-50.
- 14. Kurzen H, Esposito L, Langbein L, Hartschuh W. Cytokeratins as markers of are neoplasms with follicular differentiation: an immunohistochemical study of trichoblastoma and basal cell carcinoma. Am J Dermatopathol 2001; 23: 501-9.
- 15. Asada M, Schaart FM, Almeida Jr. HL, Korge B, Kurokawa I, Asada Y, Orfanos CE. Solid basal cell epithelioma (BCE) possibly originates from the outer root sheath of the hair follicle. Acta Derm Venereol (Stockh) 1993; 93:286-292.
- 16. Leech SN, Kolar AJ, Barret PD, Sinclair SA, Leonard N. Merkel cell carcinoma can be distinguished from metastatic small cell carcinoma using antibodies to cytokeratin 20 and thyroid transcription factor 1. J Clin Pathol 2001; 54: 727-9.
- 17. Su LD, Lowe L, Bradford CR, Yahanda AI, Johnson TM, Sondak VK. Immunostaining for cytokeratin 20 improves detection of micrometastic Merkel cell carcinoma in sentinel lymph nodes. J Am Acad Dermatol 2002; 46: 661-6.
- 18. Smeets NW, Stavast-Kooy AJ, Krekels GA, Daemen MJ, Neumann HA. Adjuvant cytokeratin staining in Mohs micrographic surgery for basal cell carcinoma. Dermatol Surg 2003; 29: 375-7.
- 19. Sasaki Y, Shimizu H, Akiyama M et al A recurrent keratin 14 mutation in Dowling-Meara epidermolysis bullosa simplex . Br J Dermatol 1999; 141: 747-8.
- 20. Liovic M, Stojan J, Bowden PE et al A novel keratin 5 mutation (K5V186L) in a family with EBS-K: a conservative substitution can lead to development of different disease phenotypes. J Invest Dermatol 2001; 116: 964-9.
- 21. Yasukawa K, Sawamura D, McMillan JR, Nakamura H, Shimizu H. Dominant and recessive compound heterozygous mutations in epidermolysis bullosa simplex demonstrate the role of the stutter region in keratin intermediate filament assembly. J Biol Chem 2002; 277: 23670-4.
- 22. D'Alessandro M, Russell D, Morley SM, Davies AM, Lane EB. Keratin mutations of epidermolysis bullosa simplex alter the kinetics of stress response to osmotic shock. J Cell Sci 2002; 115: 4341-51.
- 23. Premaratne C, Klingberg S, Glass I, Wright K, Murrell D. Epidermolysis bullosa simplex Dowling-Meara due to an arginine to cysteine substitution in exon 1 of keratin 14. Aust J Dermatol 2002; 43: 28-34.
- 24. Arin MJ, Longley MA, Anton-Lamprecht I et al A Novel Substitution in Keratin 10 in Epidermolytic Hyperkeratosis. J Invest Dermatol 1999; 112: 506-8
- 25. Arin MJ, Longley MA, Küster W et al An aspargine to threonine substitution in the 1A domain of Keratin 1: a novel mutation that causes epidermolytic hyperkeratosis. Exp Dermatol 1999; 8: 124-7.
- 26. Virtanen M, Gedde-Dahl Jr. T, Mörk NJ, Leigh I, Bowden PE, Vahlquist A. Phenotipic/ Genotipic correlations in patients with epidermolytic hyperkeratosis and the effects of retinoid therapy on keratin expression. Acta Derm Venereol 2001; 81: 163-70.
- 27. Sun XK, Ma LL, Xie YQ, Zhu XJ. Keratin 1 and 10 mutations causing epidermolytic hyperkeratosis in Chinese patients. J Dermatol Sci 2002; 29: 195-200.
- 28. Covello SP, Irvine AD, McKenna KE et al Mutations in Keratin K9 in Kindreds with Epidermolytic Palmoplantar Keratoderma and Epidemiology in Nothern Ireland. J Invest Dermatol 1998; 111: 1207-9.
- 29. Asadi AK, Kotcher LB, Jih MH. The molecular basis of hereditary palmoplantar keratodermas. J Am Acad Dermatol 2002;47:327-43.
- 30. Rugg EL, Common JEA, Wilgos A et al Diagnosis and confirmation of epidermolytic palmoplantar keratoderma by the identification of mutations in keratin 9 using denaturing high-performance liquid chromatography. Br J Dermatol 2002; 146: 952-7.
- 31. Smith FJD, Coleman CM, Bayoumy NM et al Novel Keratin 17 Mutations in Pachionychia Congenita Type 2. J Invest Dermatol 2001; 116: 806-8.
- 32. Connors JB, Rahil AK, Smith FJD, McLean WHI, Milstone LM. Delayed-onset pachyonychia congenita associated with a novel mutation in the central 2B domain of keratin 16. Br J Dermatol 2001; 144: 1058-62.
- 33. Feng YG, Xiao SX, Ren XR, Wang WQ, Liu A, Pan M. Keratin 17 mutation in pachyonychia congenita type 2 with early onset sebaceous cysts. Br J Dermatol 2003; 148: 452-5.
- 34. Hashiguchi T, Yotsumoto S, Shimada H et al A novel mutation in the keratin 17 gene in a japanese case of pachyonychia congenita type 2. J Invest Dermatol 2002; 118:545-7.
- 35. Gupta SK, Hodge WG. A new clinical perspective of corneal dystrophies through molecular genetics. Curr Opin Ophthalmol 1999; 10: 234-41.
- 36. Corden LD, Swenson O, Swenson B et al Molecular genetics of Meesmaann´s corneal dystrophy: ancestral and novel mutations in keratin 12 (K12) and complete sequence of the human KRT12 gene. Exp Eye Res 2000; 70: 41-9.
- 37. Swenson O, Swenson B, Nolle B, Rochels R, Wannke B, Thiel HJ. Mutations in the keratin gene as a cause of Meesmann-Wilke corneal dystrophy and autossomal dominant skin cornification disorders. Klin Monatsbl Augenheilkd 2000; 217: 43-51.
- 38. Ku NO, Wright TL, Terrault NA, Gish R, Omary MB. Mutation of human keratin 18 in association with cryptogenic cirrhosis. J Clin Invest 1997; 99:19-23.
- 39. Ku NO, Gish R, Wright TL, Omary MB. Keratin 8 mutations in patients with cryptogenic liver disease. N Engl J Med 2001; 344:1580-7.
- 40. Almeida Jr. HL. Genética Molecular das Epidermólises Bolhosas. An Bras Dermatol 2002; 77: 519-32.
- 41. Uitto J, Pulkkinen L. Molecular genetics of heritable blistering disorders. Arch Dermatol 2001; 137: 1458-61.
- 42. Rugg EL, Baty D, Shemanko CS et al DNA based prenatal testing for the skin blistering disorder epidermolysis bullosa simplex. Prenat Diagn 2000;20:371-7.
- 43. Pulkkinen L, Uitto J. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol 1999;18:29-42.
Publication Dates
-
Publication in this collection
09 June 2004 -
Date of issue
Apr 2004
History
-
Accepted
27 Jan 2004 -
Received
11 Dec 2003