ABSTRACT
The aim of this study was to optimize the biosynthesis of silver nanoparticles using leaves of Aegle marmelos as the primary source. The optimal reaction medium comprised 2:1 concentration of leaf extract and 6mM concentration of silver nitrate solution (pH 7. The biosynthesized silver nanoparticles were confirmed by UV-Vis spectroscopy at 420 nm, XRD and FTIR analysis. The antimicrobial properties of silver nanoparticles were confirmed with Bacillus subtilis andPseudomonas aeruginosa.
Key words: AgNO3 ; Aegle marmelos leaf; UV-Vis spectroscopy; XRD; FTIR
INTRODUCTION
Bael (Aegle marmelos (L.) Corr.) is Indian medicinal plant belonging to Rutaceae family and commonly known as wood apple, which has enormous traditional values against various diseases. From this plant, many bioactive compounds have been isolated (Badam et al. 2002; Gupta and Tondon 2004). It is used in the treatment of asthma, anaemia, fractures, healing of wounds, high blood pressure (Saswati Parichha 2004) and diabetes (Kar and Bandhopadhhyay 2003). It has hepatoprotective activity (Singanan et al. 2007), anti-inflammatory & antipyretic activity (Shankaranath 2007), antifungal activity (Patil 2009), antibacterial activity (Maheshwari 2009) and many others. The phytoconstituents of A. marmelos leaf comprise skimmianine, aegelie, lupeol, cineol, citral, citronella, cuminaldehyde, eugenol and marmesinine (Maity et al. 2009).
Nanotechnology is one of the fast developing frontiers in the field of research and technology (Mc Donell et al. 1999) and the synthesis of nanoparticles through green synthesis is gaining importance all over the World (Govindaraju et al. 2010). Silver, which is a very common and well-known metal, has gained importance due to its wide range of applications. Among the nanoparticles, silver nanoparticles have several important applications in the field of bio-labelling, sensors, antimicrobial agents and filters (Pal et al. 2007). It is also capable of destroying pathogenic bacteria by altering the cell membrane structure and functions as pesticide degrading agent. It also purifies drinking water (Liu et al. 1994; Sondi et al. 2004).
The importance of biological synthesis of nanoparticles has been realized all over the world since chemical methods are capital intensive and toxic. Thus, the need for clean, ecofriendly, cost-effective, and biocompatible synthesis of metal nanoparticles encouraged the researchers to exploit the biological sources as nanofactories (Rai et al. 2009; Seshadri et al. 2011; Wei et al. 2012). There is a need to develop eco-friendly processes (Vikas et al. 2014).
The ability of plant extracts to reduce the metal ions has been known since the early 1900s, although the nature of the reducing agents involved was not well understood. In view of its simplicity, the use of live plants or whole plant extract and plant tissue for reducing metal salts to nanoparticles has attracted considerable attention within the last 30-years (Beattie and Haverkamp 2011; Park et al. 2011;Gan and Li 2012; Kandasamy et al. 2012).
Compared with the use of whole plant extracts and plant tissue, the use of plant extracts for making nanoparticles is simpler. Plant extract mediated synthesis is an increasing focus of attention (Babu and Prabu 2011; Castro et al. 2011; Kaler et al. 2011; Lee et al. 2011; Baskaralingam et al. 2012; Daisy and Saipriya 2012).
The approach of biological synthesis of nanoparticles is not dependent on specific conditions and the rate of reaction of plant extract remains high (Bianchini et al. 2002). Silver nanoparticles are highly stable for which they are used in electronic devices to biological tools (Morgan et al. 2005). To attain greater stability, maximum yield and aggregation of particles with controlled size, it is imperative to optimize the different parameters such as the concentration of leaf extract, silver nitrate, pH and time (Morgan et al. 2005).
Thus, the aim of the present study was to optimize the synthesis of silver nanoparticles by altering the plant extract ratio with that of silver nitrate and to characterize the silver nanoparticles using UV-spec., XRD, FTIR and antimicrobial property against the pathogenic bacteria, Bacillus subtilis and Pseudomonas aeruginosa.
MATERIAL AND METHODS
The healthy leaves of Aegle marmelos (Fig. 1) were collected from the campus of VIT University, Vellore, Tamil Nadu, India.
Synthesis of Silver Nanoparticle
Healthy leaves of Aegel marmelos were washed with tap water, dried and powdered by crushing in mortar and pestle. The powdered leaves were heated with 100 mL of Milli-Q water at 65°C for 5 min and the resulting extract was filtered. From the filtrate, 10 mL was mixed with 10 mL of 1 mM silver nitrate solution and the mixture was incubated in dark at 37°C on incubator shaker. A control mixture was made by 10 mL A. marmelos leaf extract without silver nitrate solution.
Optimization of Nanoparticles Synthesis
Concentration Ratio of Leaf Extract and Silver Nitrate Solution
The concentration ratio of leaf extract and silver nitrate was optimized with the increase in concentration of leaf extract (10, 20, 30 mL) in 10 mL of 1 mM silver nitrate (ratio- 1:1,2:1,3:1). After two days of incubation, the absorbance of the resulting solution was measured spectrophotometrically.
The concentration ratio of leaf extract and silver nitrate was optimized with the increase in concentration of silver nitrate solution (2, 4, 6 and 8 mM) in constant volume of leaf extract (20 mL). Reaction mixture was incubated for two days and the absorbance of the resulting solution was measured spectrophotometrically.
Effect of the pH
The pH of the reaction was optimized by using different pH where the reaction pH was maintained at 2.0, 5.0, 7.0 and 9.0. The pH was adjusted by using 0.1 N HCl or 0.1 N NaOH. After two days of incubation, the absorbance of the resulting solution was measured spectrophoto-metrically.
Characterization of Silver Nanoparticles
Synthesis of silver nanoparticles was confirmed by taking the absorbance in UV-Vis spectra at a range 200-800 nm at a resolution of 1 nm. The lyophilized powdered sample was used for XRD (X- ray Diffraction) and FTIR (Fourier transform infrared spectroscopy) analysis. The XRD patterns were collected on broker AXS D8 Advanced X-ray diffractometer with Cu Kα radiation of wavelength 1.541º and scanning angle 2θ over the range of 10-80º. FTIR was used to characterize the nanoparticles using the lyophilized sample by KBr pellet technique in the range of 400-4000 cm-1.
Antibacterial Activity
Using the leaf extract of A. marmelos, synthesized silver nanoparticles were tested for antibacterial activity.
Well Diffusion Method
Bacillus and Pseudomonas sp. was cultured in nutrient broth for 24 h and lawn culture was made on Mueller Hinton agar (MHA) plates. Lyophilized AgNP was dissolved in sterile distilled water and sonicated. Three wells each of 5 mm diameter were made on each plate and the synthesized AgNP solution at a concentration of 60, 80 and 100 μgmL-1 was loaded in each well. The plates were then incubated at 37oC for 24 h and the zone of inhibition was calculated.
Determination of Growth Curve
Test Mycobacterium sp. was inoculated in Lowenstein-Jenson medium prepared in two side arm flask. One flask was kept as control without silver nanoparticle solution and the other with 500 µL silver nanoparticle solution. OD was checked at a gap of 1 h at 600 nm.
RESULT AND DISCUSSION
Synthesis of Silver Nanoparticles
The synthesis of the silver nanoparticles was confirmed by the characteristic color change to brown (Fig. 2) that was found in the solution containing AgNO3. The nature of the plant extract, its concentration, the concentration of the metal salt, the pH, temperature and contact time are known to affect the rate of production of the nanoparticles, their quantity and other characteristics (Dwivedi and Gopal 2010). The synthesis of silver nanoparticles using a leaf extract of Polyalthia longifolia was reported by Prasad and Elumalai (2011). Silver and gold ions could be reduced to nanoparticles using a leaf extract of Cinnamomum camphora(Huang et al. 2007). The reduction was ascribed to the phenolics, terpenoids, polysaccharides and flavones compounds present in the extract.
During the synthesis of silver nanoparticles using Annona reticulata the color of the reaction mixture, after 20 min, at room temperature, changed to dark brown, indicating the formation of AgNPs (Sivakumar and Vidyasagar 2014). The color of the Chrysanthemum morifolium extract solution containing AgNO3 changed from light yellow to yellow brown confirming the synthesis of silver nanoparticles (Yan et al. 2013).
Characterization of Silver Nanoparticles
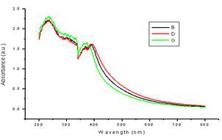
The UV-visible spectroscopy is a commonly used technique (Pal et al. 2007). Light wavelengths in the 300-800 nm are generally used for characterizing various metal nanoparticles in the size range of 2 to 100 nm (Feldheim and Foss 2002). Spectrophotometric absorption measure-ments in the wavelength ranges of 400-450 nm has been reported by Huang and Yang (2004). Characterization of silver nanoparticles was confirmed by the formation of peak between 400-420 nm range in UV-Vis spectroscopy Fig. 3) in the present study. A single Surface Plasmon Resonance (SPR) band corresponds to the spherical nanoparticles, whereas two or more SPR bands correspond to the anisotropic molecules (Krishnaraj et al. 2010). In this study, a single SPR band was exhibited by the reaction mixture, which revealed the spherical shape of the AgNPs. A characteristic peak for silver nanoparticle has been reported at 420 nm by Sivakumar and Vidyasagar (2014).
XRD is used for the phase identification and characterization of the crystal structure of the nanoparticles (Sun et al. 2000). X-rays penetrate into the nanomaterial and the resulting diffraction pattern is compared with standards to obtain structural information. The crystalline nature of AgNPs was confirmed by the X-ray diffraction studies (Fig. 4).
FTIR spectroscopy is useful for characterizing the surface chemistry (Chithrani et al. 2006). Organic functional groups (e.g., carbonyls, hydroxyls) attached to the surface of nanoparticles and the other surface chemical residues are detected using FTIR. FTIR spectrum of AgNPs is shown in the Figure 5. It revealed the possible biomolecules present in the leaf extract, which was accountable as the reducing agent for the silver ions and its interaction with the AgNPs. The IR spectrum showed an intense band at 344.01 cm-1, which corresponded to the strong stretching vibrations of hydroxyl group (-OH) of phenolic compounds (Yan et al. 2013).
Optimization of Silver Nanoparticle Synthesis
Concentration of Leaf Extract
Different concentration of leaf extract was optimized and the maximum silver nanoparticle synthesis occurred in 2:1 ratio, which was further confirmed by the formation of highest peak in spectroscopy and brown color formation. Thus, this ratio was considered as optimum and the next parameter was performed based on this ratio and color change (Fig. 6). The ratio varied from plant to plant; for example, in the leaf of P. guajava plant, a combination of 1.0 mL + 9.0 mL showed highest peak at 440 nm (Vikas et al. 2014). In the synthesis of silver nanoparticles using a geranium (Pelargonium graveolens) leaf extract, the particles formed rapidly and a stable size of 16-40 nm was achieved (Shankar et al. 2003).
UV-vis spectroscopy results showing concentration of leaf extract Vs. silver nano particles synthesis. (G-2:1, D-1:1, B-3:1).
Concentration Ratio of Silver Nitrate Solution
Results showed that 6 mM concentration of silver nitrate gave the maximum formation with the absorbance peak at 400-420 nm (Fig. 7) and color turned brown (Fig. 2) after 48 h incubation. At 1 mM, silver nitrate gave characteristic absorption peak at 440 nm in the UV-vis spectrum forP. guajava leaf extract (Vikas et al. 2014). The intensity of absorption spectra of AgNP increased with increasing the concentration of AgNO3 (1 to 5 mM); 4 Mm concentration of AgNO3 solution showed narrow size distribution of AgNPs using O. tenuiflorum leaf extracts as a reducing agent (Dulen 2014).
UV-vis spectroscopy results showing concentration of silver nitrate Vs. silver nano particles synthesis(F-6 mM, D-4 mM, B-2 mM).
pH
pH is considered as important parameter in silver nanoparticle synthesis. The solution was adjusted in different pH and the concentration was kept at 6 mM in 2:1 ratio. Minimum silver nanoparticles formation was found at acidic pH (2.0 and 5.0) and alkaline pH (9.0). pH 7.0 was optimum with maximum nanoparticle synthesis. Absorbance peak was highest at 400-420 nm (Fig. 8). Similarly in the study of Vikas et al. (2014), the AgNP synthesis using P. guajava leaf extract, pH 7.0 was optimum for the synthesis of the nanoparticles.
UV-vis spectroscopy results showing different pH Vs. silver nano particles synthesis. (F-7, H-5, D-9, B-2).
Antibacterial Activity
Silver nanoparticles have been commonly found to have broad spectrum antimicrobial activity against human and animal pathogens (Arulkumar and Sabesan 2010; Ali et al. 2011). The antimicrobial activity of synthesized silver nanoparticles was confirmed by relative zone of inhibition (Table 1, Figs 9 and 10 ). The antimicrobial activity of the silver nanoparticle synthesized from A. marmelos had sensitivity against E. coli, P. aeuruginosa and S. aureus (Yamini et al. 2014). The impregnated disc with A. marmelos AgNPs exhibited lesser inhibition compared to that of the direct addition into the well. The AgNPs synthesized from the stem bark ofAdansonia digitata (L.) showed highest inhibiting effect onP. vulgaris, followed by E. coli, P. aeruginosa, K. pneumonia, S. typhimurium, B. subtilis and S. aureus as confirmed by the diameter of zone of inhibition (Maruti et al. 2015). Patil et al. (2012 a,b) produced highly stabilized silver nanoparticles (25-40 nm) using a leaf extract of O. tenuiflorum. Krishnaraj et al. (2010) synthesized silver nanoparticles (20-30 nm) using a leaf extract of Acalypha indica. The nanoparticles showed antimicrobial activity against water-borne pathogens such as E. coli and Vibrio cholerae. Spherical silver nanoparticles (40-50 nm) have been produced using a leaf extract ofEuphorbia hirta (Elumalai et al. 2010). Silver nanoparticles produced using peel extract ofCitrus sinensis showed a broad spectrum antibacterial activity (Kaviya et al. 2011b). The particles formed at 60°C had an average size of around10 nm but reducing the reaction temperature to 25°C increased the average size to 35 nm (Kaviya et al. 2011b). Gram positive bacterium S. aureus showed highest activity compared to Gram negative bacterium Aeromonas hydrophila in silver nanoparticles synthesized by P. guajava leaf extract (Vikas et al. 2014). The silver nanoparticles causes an increase in cell membrane permeability and results in cell death (Guzman et al. 2008).
Determination of Growth Curve
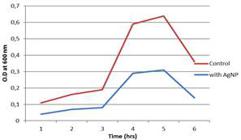
Antibacterial activity of silver nanoparticles was determined using the growth curve method, which showed relative decrease in OD containing AgNPs reducing the growth of both B. subtilis and P. aeruginosa; in the absence of AgNps, the OD of both bacterial cultures increased steadily, indicating rapid bacterial growth (Fig. 11).
Similar study with Vitis vinifera was carried out where the presence of AgNPs reduced the growth of B. subtilis andE. coli indicated by the decreased OD value (Kaushik et al. 2013). The optimum harvest time for the cells of Fusarium oxysporum in the presence of the enzyme inducer AgNO3 (0.1 mM) was between 28 to 32 h (Korbekandi et al. 2013).
CONCLUSION
In this work, silver nanoparticles were synthesized with greater stability using simple low cost and eco-friendly biological approach. This procedure could be highly suitable for large-scale production as it is very rapid and shortens the duration of many bio-based applications. The use of plant extracts for making metallic nanoparticles is inexpensive, easily scaled-up and environmentally benign. It is especially suited for making the nanoparticles that must be free of toxic contaminants as required in therapeutic applications. The plant extract-based synthesis can provide the nanoparticles of a controlled size and morphology. Thus, silver nanoparticles could become one of the most common metals used in medical treatment.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the XRD and FTIR facilities sponsored by the DST - FIST, Govt. of India.
References
- Ali DM, Thajuddin N, Jeganathan K, Gunasekaran M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B Biointerfaces2011;85:360-365.
- Arulkumar S, Sabesan M. Biosynthesis and characterization of gold nanoparticle using antiparkinsonian drug Mucuna pruriens plant extract. Int Res Pharm Sci. 2010;1: 417-420.
- Babu SA, Prabu HG. Synthesis of AgNPs using the extract ofCalotropis procera flower at room temperature. Mater Lett 2011;65:1675-1677.
- Badam L, Bedekar SS, Sonawane KB, Joshi SP. In vitro antiviral activity of Bael (Aegle marmelosCorr.) upon human Cox sackiviruses B1-B6. J Commun Dis. 2002; 34: 88.
- Baskaralingam V, Sargunar CG, Lin YC, Chen JC. Green synthesis of silver nanoparticles through Calotropis gigantea leaf extracts and evaluation of antibacterial activity against Vibrio alginolyticus Nanotechnol Dev 2012;2:e3.
- Beattie IR, Haverkamp RG. Silver and gold nanoparticles in plants: sites for the reduction to metal. Metallomics 2011; 3:628-632.
- Bianchini A, Bowles KC, Brauner CJ, Gorsuch JW, Kramer JR, Wood CM. Evaluation of the effect of reactive sulfide on the acute toxicity of silver (I) to Daphnia magna Part 2. Toxicity results. Environ Toxicol Chem. 2002; 21: 1294-1300.
- Bianchini A, Grosell M, Gregory SM, Wood CM. Acute silver toxicity in aquatic animals is a function of sodium uptake rate. Environ Sci Technol. 2002; 36: 1763-1766.
- Castro L, Blázquez ML, Munoz JA, Gonzalez F, García-Balboa C, Ballester A. Biosynthesis of gold nanowires using sugar beet pulp. Process Biochem. 2011;46:1076-1082.
- Ch MarutiKesava Kumar, Yugandhar P, Suhrulatha D, Savithramma N. Synthesis, Characterization and Antimicrobial Studies of Stem Bark Mediated Synthesis of Silver Nanoparticles From Adansonia digitata (L.). J. Pharm Sci Res2015; 7(2): 76-82.
- Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662-668.
- Conrad AH, Tramp CR, Long CJ, Wells DC, Paulsen AQ, Conrad GW. Ag+ alters cell growth, neurite extension, cardiomyocyte beating, and fertilized egg constriction. Aviat Space Environ Med1999; 70:1096-1105.
- Daisy P, Saipriya K. Biochemical analysis of Cassia fistulaaqueous extract and phytochemically synthesized gold nanoparticlesas hypoglycemic treatment for diabetes mellitus. Int J Nanomedicine 2012; 7: 1189-202.
- Dulen Saikia. Green synthesis and optical characterizations of silver nanoparticles. IJLRST2014; 3(2): 132-135.
- Dwivedi AD, Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A 2010;369:27-33.
- Elumalai E, Prasad T, Hemachandran J, Therasa SV, Thirumalai T, David E. Extracellular synthesis of silver nanoparticles using leaves ofEuphorbia hirta and their antibacterial activities. J Pharm Sci Res 2010; 2:549-554.
- Elumalai E, Prasad T, Hemachandran J, Therasa SV, Thirumalai T, David E. Extracellular synthesis of silver nanoparticles using leaves ofEuphorbia hirta and their antibacterial activities. J Pharm Sci Res2010; 2:549-554.
- Feldheim DL, Foss CA. Metal nanoparticles: synthesis, characterization, and applicationsBoca Raton, FL: CRC Press; 2002.
- Gan PP, Li SFY. Potential of plant as a biological factory to synthesize gold and silver nanoparticles and their applications. Rev Environ Sci Biotechnol 2012; 11: 169-206.
- Govindaraju K, Tamilselvan S. Silver nanoparticles bySolanum torvum and their promising antimicrobial activity. J Biopest. 2010; 3(1): 394-399.
- Guggenbichler JP, Boswald M, Lugauer S, Krall T. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection. 1999; 27(Suppl):S16-S23.
- Gupta AK, Tondon N. Review on Indian medicinal plants", Indian council of medicinal research, New Delhi, 2004; 312.
- Hassan Korbekandi, Zeynab Ashari, Siavash Iravani and Sajjad Abbasi. Optimization of Biological Synthesis of Silver Nanoparticles usingFusarium oxysporum IJPR 2013; 12 (3): 289-298.
- Hirasawa F, Kawarada Y, Sato M, Suzuki S, Terada K, Miura N, et al. The effect of silver administration on the biosynthesis and the molecular properties of rat ceruloplasmin. Biochim Biophys Acta 1997; 1336: 195-201.
- Huang H, Yang X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydrate Res 2004;339:2627-2631.
- Huang JL, Li QB, Sun DH, Lu YH, Su YB, Yang X, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnol 2007;18.
- Inbakandana D, Kumar C, Stanley Abraham L, Kirubagaran R, Venkatesan R, Ajmal Khan S. Silver nanoparticles with anti microfouling effect: A study against marine biofilm forming bacteria. Colloids and Surfaces B: Biointerfaces. 2013; 111: 636-643.
- Ivan Sondi and Branka Salopek-Sondi. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interf Sci 2004; 275 : 177-182.
- Jayabrata DasPalaniyandi Velusamy. Biogenic synthesis of antifungal silver nanoparticles using aqueous stem extract of banana. Nano Biomed Eng. 2013; 5(1).
- Kaler A, Nankar R, Bhattacharyya MS, Banerjee UC. Extracellular biosynthesis of silver nanoparticles using aqueous extract of Candida viswanathii J Bionanosci 2011; 5: 53-58.
- Kandasamy K, Alikunhi NM, Manickaswami G, Nabikhan A, Ayyavu G. Synthesis of silver nanoparticles by coastal plant Prosopis chilensis (L.) and their efficacy in controlling vibriosis in shrimpPenaeus monodon Appl Nanosci 2012.
- Kar A, Choudhry BK, Bandhopadhyay NG. Comparative evaluation of hypoglycemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol. 2003; 84: 105-108.
- Kaushik Roy, Supratim Biswas, PatakiC Banerjee. 'Green' Synthesis of Silver Nanoparticles by Using Grape (Vitis vinifera) Fruit Extract: Characterization of the Particles and Study of Antibacterial Activity. Research J Pharma Biol Chem 2013; 4(1): 271-278.
- Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K. Biosynthesis of silver nanoparticles using Citrus sinensispeel extract and its antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 2011b;79:594-598.
- Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids and Surfaces B: Biointerfaces 2010; 76 (1): 50-56.
- Lee HJ, Lee G, Jang NR, Yun JH, Song JY, Kim BS. Biological synthesis of copper nanoparticles using plant extract. Nanotechnol 2011;1:371-374.
- Liu Z, Stout JE, Tedesco L. Controlled evaluation of copper-silver ionization in eradicating Legionella pneumophila from a hospital water distribution system. J Infect Dis 1994;169:919-922.
- Maheshwari VL, Joshi PV, Patil RH. In vitro anti diarrhoeal activity and toxicity profile of Aegle marmelosCorrea ex. Roxb. dried fruit pulp", Natural Product Rad. 2009; 8 (5): 498-502.
- Maity P, Hansda D, Bandyopadhyay U, Mishra DK. Biological activities of crude extracts of chemical constituents of Bael, Aegle marmelos (L.) Corr. Indian J Exp Biol. 2009; 47: 849-861.
- MaribelG Guzmán, Jean Dille, Stephan Godet. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. WASET. 2008; 43.
- McDonell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999; 12 (1): 147-179.
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli Appl Environ Microbiol 2007;73:1712-1720.
- Park Y, Hong YN, Weyers A, Kim YS, Linhardt RJ. Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol 2011;5:69-78.
- Patil SV, Borase HP, Patil CD, Salunke BK. Biosynthesis of silver nanoparticles using latex from few euphorbian plants and their antimicrobial potential. Appl Biochem Biotechnol 2012a;167:776-790.
- Patil R, Kokate M, Kolekar S. Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuifl orum leaf extract and their antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 2012b; 91:234-238.
- Patil RH, Chaudhary B, Settipalli S. Antifungal and Antiaflatoxigenic activity of Aegle marmelos Linn.Pharmacog J 2009; 1: 4.
- Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 2009; 27(1): 76-83.
- Sachin Seshadri K, Saranya Meenal Kowshik. Green synthesis of lead sulfide nanoparticles by the lead resistant marine yeast, Rhodosporidium diobovatum ACS Publications. 2011; 21:1086-1090.
- Saswati Parichha. Bael (Aegle Marmelos): Nature's Most Natural Medicinal Fruit", Orissa Review. 2004.
- Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog 2003;19:1627-1631.
- Shankarnanth V, Balakrishnan N, Suresh D, Sureshpandian G, Edwin E, Sheeja E. Analgesic activity of methanol; extract of Aegle marmelos leaves. Fitoterapia, 2007; 78 (3): 258-259.
- Shivakumar Singh P, Vidyasagar GM. Biosynthesis, Characterization, and Antidermatophytic Activity of Silver Nanoparticles Using Raamphal Plant (Annona reticulata) Aqueous Leaves Extract. Indian J Mater Sci. 2014; 5.
- Singanan V, Singanan M, Begum H. The hepatoprotective effect of bael leaves (Aegle marmelos) in alcohol induced liver injury in albino rats. Int J Sci Technol. 2007; 2(2): 83-92.
- Sukdeb Pal, Yu Kyung Tak, and Joon Myong Song. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl Environ Microbiol 2007; 73(6): 1712-1720.
- Sun S, Murray C, Weller D, Folks L, Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000; 287:1989-1992.
- Tammie P Morgan, Christine M Guadagnolo, Martin Grosell, Chris M Wood. Effects of water hardness on toxicological responses to chronic waterborne silver exposure in early life stages of rainbow trout (Oncorhynchus mykiss).. Environ Toxicol Chem 2005; 24 (7): 1642-1647.
- Vikas CS, Bajaj S, Sharma B. Microwave-mediated green synthesis of silver nanoparticles by using Vinca rosea & its application in water pollution control. Indian J Sci Res. 2014; 4 (1): 145-148.
- Vikas Sarsar, Manjit K Selwal, Krishna Kumar Selwel. Significant parameters in the optimization of biosysnthesis o silver nanoparticles usingPsidium guajava leaf extract and evaluation of their antimicrobial activity against human pathogenic bacteria. Int J Adv Pharma Sci. 2014; 5(1): 1769-1775.
- Wei X, Luo M, Li W, Yang L, Liang X, Xu L, et al. Synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3 Bioresource Technol. 2012;103:273-278.
- Yamini Sudha Lakshmi S, Fouzia Banu, Brindha V, Gopalakrishnan S, Gajendran N. Antimicrobial activity of Aegle marmelos (Correa) Linn. Silver nanoparticles. In J Drugs Diseases2014; 3 (1).
- Yan He, Zhiyun Du, Huibin Lv, Qianfa Jia Zhikai Tang, Xi Zheng, Kun Zhang, Fenghua Zhao. Green synthesis of silver nanoparticles byChrysanthemum morifolium Ramat. Extract and their application in clinical ultrasound gel. Int J Nanomedicine 2013; 8: 1809-1815.
Publication Dates
-
Publication in this collection
Sep-Oct 2015
History
-
Received
04 May 2015 -
Accepted
17 June 2015

 Optimization of Parameters for Biosynthesis of Silver Nanoparticles Using Leaf Extract of Aegle marmelos
Optimization of Parameters for Biosynthesis of Silver Nanoparticles Using Leaf Extract of Aegle marmelos