Abstract
Powder from jambolan fruit is rich in bioactive compounds, such as pigments, and they present the potential to be used as a food colorant. This study aims to evaluate color and total anthocyanin content (TAC) and stability in freeze-dried powder of jambolan fruits at four different degrees of maturity during storage in low-density polyethylene (LDPE) and metalized films at room temperature, thus simulating the storage after opening the package. The powder of fully mature fruits showed the highest anthocyanin content, evidenced by its intense purple color, as well as the highest anthocyanin degradation rate during storage after opening the package. The metalized film showed an anthocyanin concentration loss of 44%, however, the loss was 56% for the LDPE film. Immature fruits became darker, whereas the matured ones kept stable lightness within 90 days. The hygroscopicity increased with maturation, being related to the stability of anthocyanins with storage time. The metalized packaging assured stronger color and anthocyanin protection, besides enabling lower moisture gain in jambolan powders than LDPE packaging.
Keywords: Lyophilization; Java plum; Jamun; Anthocyanins; Color; Food quality; Hygroscopicity
Resumo
Produto em pó de frutos de jambolão é rico em compostos bioativos, como os pigmentos, apresentando potencial para ser usado como corante alimentício. O objetivo deste estudo é avaliar a estabilidade da cor e o conteúdo de antocianinas em pó de fruto liofilizado de jambolão em diferentes estádios de maturação, durante 90 dias de armazenamento em temperatura ambiente, em embalagens transparente (PEBD) e metalizada, simulando o armazenamento após abertura das embalagens. A cor roxa se intensificou com o amadurecimento do fruto. Pós dos frutos maduros apresentaram maior teor de antocianinas, evidenciado pela cor roxa mais intensa, assim como maior taxa de degradação durante o armazenamento após abertura das embalagens. Filmes metalizados resultaram em redução do conteúdo de antocianinas de 44%, enquanto esta perda foi de 56% para os filmes transparentes. Frutos imaturos escureceram, enquanto frutos maduros mantêm a luminosidade estável após 90 dias de armazenamento. A higroscopicidade aumentou com a maturação e está relacionada com a estabilidade das antocianinas com o tempo de armazenamento. A embalagem metalizada assegurou maior proteção às antocianinas e à cor, além de proteção ao ganho de umidade no produto, quando comparada à embalagem transparente.
Palavras-chave: Criodessecação; Jamelão; Antocianinas; Cor; Qualidade de Alimentos; Higroscopicidade
Highlights
• Powder from fully matured jambolan fruits showed the highest anthocyanins degradation rate during storage of previously opened packages.
• Hygroscopicity increased with maturation and correlates with lower stability of anthocyanins with storage time.
• Metalized film packages showed better protection against anthocyanins degradation than low-density polyethylene.
1 Introduction
The interest in using jambolan (Syzygium cumini (L.)) fruits and extracts has been growing fast in the last decades due to their several benefits to human health. The fruit is composed of several substances, which present nutraceutical and functional properties, such as antioxidant, anticancer, antimicrobial and antidiabetic activity, as well as cardiovascular protection (Ayyanar & Subash-Babu, 2012; Benherlal & Arumughan, 2007; Faria et al., 2011; Grover et al., 2000; Longo et al., 2007; Menezes et al., 2013; Santos et al., 2020; Sharma et al., 2016; Singh et al., 2016; Vikrant et al., 2001).
The composition of bioactive compounds in fruits varies at different degrees of maturity. Unripe fruits present high hydrolyzable tannins (gallo- and ellagitannins, and ellagic and gallic acids) and flavonoid contents, which make them suitable for nutraceutical use. On the other hand, the contents of hydrolyzable tannins decrease and anthocyanins increase as the ripening process progresses. Anthocyanins are responsible for the typical purple color of jambolan fruits (Lestario et al., 2017; Patel & Rao, 2014). Therefore, dried products from anthocyanin-rich mature fruits could be recommended as a food colorant, consisting of the advantage of presenting biological activities (Bezerra et al., 2015; Freitas-Sá et al., 2018).
Several drying processes such as convective drying in a Fixed or Fluidized Bed Dryer (FBD), Freeze-Drying (FD), Foam-Mat Drying (FMD), and Spray Drying (SD) are reported to enable the commercialization of dried fruit and extracts (Borges et al., 2016; Iasnaia Maria de Carvalho et al., 2017; Mussi et al., 2015; Santiago et al., 2016; Soares & Pereira, 2020). The drying method and process conditions influence both the degradation of unstable compounds and the extraction efficiency. Freeze-drying is recognized as a process that can minimize the degradation of unstable compounds during processing compared to other drying methods, mostly due to low-temperature exposure (Cheng et al., 2017). Though, it yields high-quality products and is often used as a reference method (Franks, 1998; Waghmare et al., 2021). (Nemzer et al., 2018) reported that freeze-drying exhibited better retention of anthocyanins, vitamin C, phenolics, and Oxygen Radical Absorbance Capacity (ORAC) antioxidant capacity in several berries than other drying methods. In addition, it can enhance the extraction efficiency of bioactive compounds probably due to structural changes during the process (Eliasson et al., 2017).
However, some degradation of bioactive compounds during freeze-drying may occur, and it can be related to higher oxygen exposure due to the porous structure formed during freeze-drying (Fujita et al., 2013; Hazarika & Gosztola, 2020). (Fujita et al., 2013) reported that freeze-drying preserved the content of proanthocyanidins and decreased ascorbic acid by 18% with respect to camu-camu powders, whereas Spouted Bed Dryers (SBDs) showed a decrease of 45-64% in ascorbic acid and 15.5-18.4% in proanthocyanins. In fact, anthocyanins may present instability depending on temperature, oxygen, moisture, and light (Ayyanar & Subash-Babu, 2012; Cavalcanti et al., 2011; Damodaran et al., 2007; Hendry & Houghton, 1992; Sharma et al., 2016; Veigas et al., 2007). Color is an important food quality attribute, besides being associated with fruit ripeness due to the presence of pigments (Damodaran et al., 2007; Usenik et al., 2009). The structure of anthocyanins and, consequently, their color, may change depending on factors affecting its stability during processed-food storage. Thus, drying method, storage conditions and dry-food packaging may affect products’ shelf life, which is controlled by properties of the food itself, as well as by specific barrier properties of each packaging (Wrolstad, 2004; Tonon et al., 2010; Zorić et al. 2016; Udomkun et al., 2016; Sinela et al., 2017). Freeze-dried bayberry powders showed better retention of total polyphenols, gallic acid, protocatechuic acid, cyanidin-3-o-glucoside, and anthocyanins during storage than spray dried ones (Cheng et al., 2017).
The authors did not conduct a previous study about the storage stability of jamolan fruit powder at different maturation despite the health-beneficial effects of jambolana fruit consumption and the changes in fruit composition during maturation and storage. Therefore, the current study aimed to evaluate the color and TAC stability of freeze-dried edible fractions of jambolan fruits at different degrees of maturity during storage at room temperature in transparent and metalized packaging after opening the package.
2 Materials and methods
2.1 Raw material
Jambolan fruits were manually collected from trees located at the University campus in Campos dos Goytacazes, in the state of Rio de Janeiro, in Brazil, from February to March 2016. Fruits were sorted into four different degrees of maturity based on visual observation of peel color. The aforementioned degrees of maturity were green (D1), green-pink (D2), pink-light-purple (D3), and purple (D4). The selected fruits were washed in tap water, immersed in 100 mg.L-1 sodium hypochlorite solution for 15 min, rinsed in distilled water (once), and dried at room temperature (25 ºC). The whole fruits were stored in plastic bags at -18 ºC for one month before the edible fractions (consisting of pulp and peel) were manually separated from the seeds with a sharp knife. Right after the separation, the edible fractions were stored at -18 °C in individual portions for further drying and analysis.
2.2 Freeze drying
The edible portions of fruits at all maturation degrees were freeze-dried in a benchtop manifold freeze dryer (LIOTOP, L202, Brazil) until a constant weight was reached. Freeze-drier vessels were wrapped in aluminum foil to protect the samples from light during the process. Moisture content and water activity in the initial and dried samples were measured. The moisture content was determined by the AOAC Method 925.23 (Association of Official Analytical Chemists, 1998), i.e., samples were dried in an oven at 105 °C for 24 h. Water activity was determined in a dew point water activity meter at 25 °C (Aqualab 4 TEV, Decagon, USA). After the freeze-drying, samples were grounded for 20 s in a stainless-steel electric coffee grinder (PC-KSW 1021, ProfiCook, Germany) adapted with external insulation, which consisted in a frozen gel bag. The ground sample size was homogenized through a 0.71 mm Tyler sieve (mesh 25) to obtain a powder with particle diameters smaller than 0.71 mm.
2.3 Hygroscopicity
The hygroscopicity was determined based on previous publications (Cai & Corke, 2000; Tonon et al., 2008). An aluminum pan with 1.0 g of sample was placed in a desiccator containing NaCl saturated solution (water activity 0.75) at 25 °C. After 1-week samples were dried until constant weight at 105 °C. The hygroscopicity was expressed as the mass of absorbed moisture per 100 g of dry solids.
2.4 Color
The CIE Lab color space parameters of powder samples were measured in a colorimeter (MiniScan XE Plus, HunterLab, USA) following manufacturer instructions. The color difference (ΔE*), the cylindrical coordinates, chroma (C*) and hue angle (hab) resulted from L*-, a*- and b*- values, according to Equations 1, 2, and 3, respectively (Konica Minolta, 1998).
wherein L0*, a0* and b0* represented the color values of the initial samples and L*, a*, and b* represented the color of samples at t storage time. Ten grams of powdered sample were poured into a glass vessel (64 mm diameter) covered with a black lid to avoid influence from external light. Readings were conducted every 90° of vessel rotation (horizontal plane). This procedure was repeated at least three times for every sample after powder homogenization.
2.5 Total anthocyanins
The anthocyanin extraction consisted of extracting 0.5 g of samples using 5 mL of methanol acidified with HCl to pH 2.0. The extraction was performed as follows: agitation in a vortex for 5 min at 120 rpm; sonication for 30 min; and centrifugation for 10 min at 1100 rpm (mod 8BT, ITR, Brazil). Then, samples were kept for 24 h at 5 °C and protected from light to complete anthocyanin extraction. They were centrifuged for 10 min after 24h of cold extraction. The extract was centrifuged for 10 min at 110 rpm and the supernatant was pipetted for anthocyanin determination. Total anthocyanins were quantified based on the pH-differential method (Lee et al., 2005), using a UV-visible spectrophotometer (ThermoScientific, Genesys 105 UV-VIS, China) at 520 and 700 nm. The anthocyanin content (mg 100 g-1 dry matter) was expressed as cyaniding-3-glucoside equivalents by taking into consideration the molar extinction coefficient 26,900 and the molecular weight 449.2 g mol -1.
2.6 Packaging and storage stability
Powder samples (14 g) at four degrees of maturity were stored in sealed packaging (made of two different materials) at room temperature (25 ± 2 °C) after the drying process. The transparent bag was made of low-density polyethylene (LDPE) film 0.035 (± 0.001) mm thick (Talge, Brazil). The laminated pouch (Metalized) had a film thickness of 0.125 (± 0.001) mm and comprised an external laminated polyester layer and an internal polyethylene layer (TradPouch, Tradbor, Brazil).
Samples were withdrawn from the packaging for color measurement at time intervals (7, 14, 21, 30, 45, 60, and 90 days) until 90 days of storage. After the color-reading, a 0.5 g aliquot was taken for anthocyanin determination, whereas the remaining powder was placed back in the packaging to continue the storage. This procedure subjected samples to environmental conditions, i.e., oxygen, humidity, and temperature, simulating storage by consumers. Additionally, the water activity in the samples was measured at the 60th and 90th storage days to assess moisture gain during storage.
The anthocyanin stability during storage was considered as a first-order kinetic reaction. Data of TAC versus time were fitted to Equation 4 to estimate first-order kinetic rate constant (k) and half-life (t1/2) (Equation 5).
wherein C is the TAC (mg 100 g-1) at storage time t (min) and C0 is the initial TAC.
2.7 Statistics
All analyses were repeated at least three times and values were expressed as average ± standard deviation. The experimental data of the physical-chemical analyses and the color of the four degrees of maturity were subjected to Analysis of Variance (ANOVA) and Tukey’s test in the Basic software (XLStat, USA). The average values were considered to be significantly different at a 95% confidence level (p ≤ 0.05). The anthocyanin degradation kinetics data fitting was done by nonlinear regression and evaluated by the determination coefficient (R2).
3 Results and discussion
3.1 Characterization of freeze-dried jambolan edible fractions
Table 1 presents moisture and anthocyanin contents in edible fractions of jambolan fruits at the four degrees of maturity. The moisture content and hygroscopicity of freeze-dried products were lower in the edible fraction of green fruits and higher in the ripest ones (purple-black). Final moisture content linearly was correlated with hygroscopicity (R2 = 0.99) and maturation (R2 = 0.96). Previous studies reported water accumulation and increased sugar content in fruits throughout maturation, with an intense increase of glucose and fructose contents (Mussi, 2018; Sturm et al., 2003). Thus, water molecules may be strongly bonded to sugars, and to the cellular structure as maturation progressed. This hinders the water loss during freeze-drying due to high viscosity, and low vapor pressure, diffusion, and mobility of watery solution of fruit tissue (Maltini et al., 2003), thus increasing final moisture and hygroscopicity of more mature fruits.
Moisture, hygroscopicity, total anthocyanin contents, and color of jambolan powder at four degrees of maturity.
As expected, the TAC increased as the fruit maturation progressed; such pigment was not observed in green fruits (D1). The evolution in anthocyanin formation presented an exponential character since there was an abrupt anthocyanin increase in the last degree of maturity. Similar results were reported by Lestario et al. (2017), who showed that the anthocyanin content increased, and its composition changed during fruit maturation. All color parameters presented statistical differences between powders from jambolan fruits at different degrees of maturity (p ≤ 0.05). The fruit powders became darker (decreased L*-values) and presented a more red color (increased a*-values) as the maturation progressed (Figure 1). The b-values indicated that the color of the fruit powders changed from less yellow to more blue (decreased b*-values). Both a* and b*-values contributed to the purple color characteristic of the fully-ripe fruit powders (D4), with the hue moving from yellow-orange to purple values (h*ab).
Visual observation of fruits and freeze-dried jambolan powders from different degrees of maturity stored in low-density polyethylene (LDPE) and metalized packing. Maturity degree: Green (D1); Green-pink (D2); Pink-light purple (D3) and Purple-black (D4)
3.2 Effect of maturation degree and storage conditions on moisture gain
Due to hygroscopic character, water activity increased in all samples after opening the package during storage in both packaging materials in comparison to the initial samples (data not shown). However, powders stored in metalized packaging showed a lower increase in water activity than the ones stored in LDPE bags, as expected (p ≤ 0.05) (Table 2). Water activity increased by 3.4 and 3.0 times, on average, in samples packed in LDPE and metalized films, respectively. The protection against moisture is affected by the nature of the packaging material and by layer thickness (Zorić et al. 2016). Similar results were reported by (Henríquez et al., 2013), who observed higher moisture gain in apple peel powders stored in high-density polyethylene than in high-barrier metalized film pouches. Water gain plays a key role in the quality and shelf-life of dried powders. Powders from fully mature fruits (D4) showed agglomeration within 90 storage days, because of water gain after opening the package. Thus, a low relative humidity atmosphere is needed to handle and store those products.
Water activity of jambolan powder at four degrees of maturity after storage in low-density polyethylene (LDPE) and metalized packing.
3.3 Anthocyanin stability
Anthocyanins are the main pigment responsible for the color of jambolan fruits and show increased content as ripening progresses, as mentioned previously (Figure 1). Therefore, powders from the most immature fruits (D1) were not evaluated for anthocyanin stability during storage, as anthocyanins are not present in their composition.
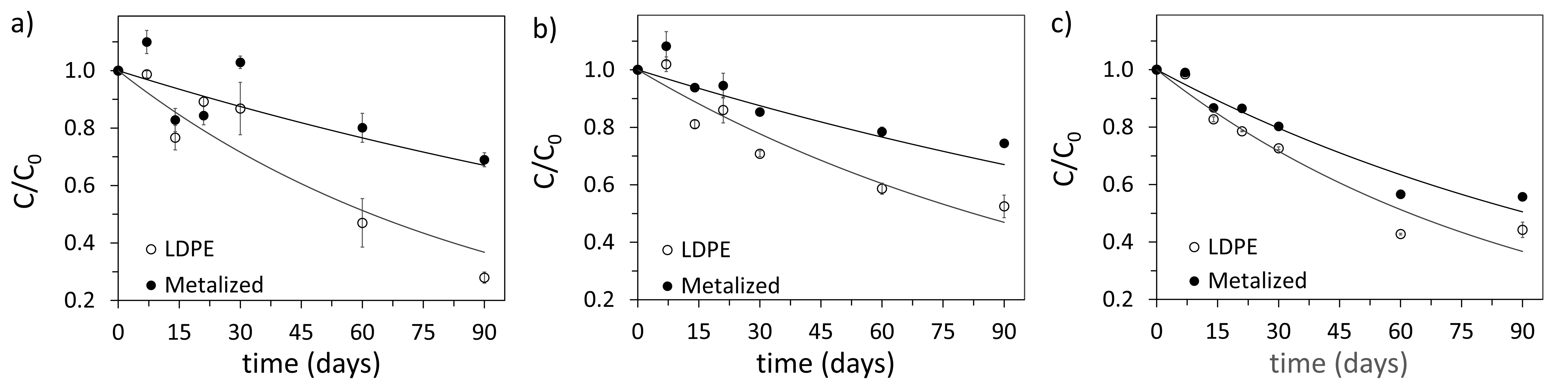
Anthocyanin degradation data fitted well to the first-order kinetics model (Equation 4) (R2≥0.91) (Table 3), except for samples from the green-pink (D2) degree of maturity. The model explained 88% and 62% of anthocyanin data for D2 samples in the LDPE and metalized packaging types, respectively. Even though, the model was considered and was used to express the trend of anthocyanins stability for powders from different degrees of maturity during storage in two different packings.
First-order kinetics parameters for anthocyanin degradation during storage of jambolan powder at three degrees of maturity in LDPE and metalized packing.
As expected, the metalized packaging provided stronger protection against anthocyanin degradation than the LDPE one throughout the storage of all samples (Figure 2). The half-life of samples stored in the metalized packaging was 2.5, 2.3, and 1.5 times higher than in the LDPE one at the green-pink (D2), pink-light purple (D3), and purple-black (D4) degree of maturity, respectively. However, those values were considerably lower than the one found for foam mat-dried jambolan juice powder stored at 25 ºC (Tavares et al., 2020). The authors suggest that this high stability can be attributed to the maltodextrin protective effect, which is used in the foam formulation.
Kinetics of anthocyanin degradation of jambolan powders from different degrees of maturity stored in low-density polyethylene (LDPE) and metalized packing. Maturity degrees: (a) Green-pink (D2); (b) Pink-light purple (D3); and (c) Purple-black (D4). The error bars represent the standard error of average values.
It is well known that light, oxygen, and moisture content accelerate anthocyanin degradation during storage (Gradinaru et al., 2003; Sari et al., 2012; Sharma et al., 2016; Wrolstad, 2004). The anthocyanin concentration losses were lower than 2% after 7 days of storage when the packages were first opened, showing some accordance with the reported stability of jambolan foam-mat dried powders (Iasnaia Maria de Carvalho et al., 2017). As previously mentioned, our samples presented hygroscopic character and the samples absorbed water from the environment after several package openings. Thus, the losses might be related to the increase in water activity. In fact, the metalized packaging provided stronger protection against moisture migration during storage. Anthocyanin concentration showed statistical differences after 90, 30, and 14 days of storage between samples stored in LDPE and metalized packages (p ≤ 0.05), for green-pink (D2), pink-light purple (D3), and purple-black (D4) degree of maturity, respectively.
Fully mature samples (D4) showed the highest anthocyanin degradation rate, achieving 44% and 56% loss of anthocyanin concentration after 90 days (p ≤ 0.05), similar to results reported by (Braga & Rocha, 2015) for milk-blackberry pulp powder and (Cheng et al., 2017) for freeze-dried and spray-dried bayberry powder. The anthocyanin content of this sample (D4) (before storage) was 97 and 77% higher than the ones from maturity degree D2 and D3, respectively (Table 1). Higher TAC may favor more intense degradation reactions than lower contents since the reaction rate is proportional to reactant concentrations (Damodaran et al., 2007). At the same time, higher hygroscopicity, fruit composition (sugars, ascorbic acid, other organic acids, and minerals), and the profile of anthocyanins and other polyphenolics can explain the higher anthocyanin reduction in powders from purple-black samples throughout storage.
Anthocyanin degradation reaction produces brown pigments (Skrede et al., 2000; Wrolstad, 2004) or colorless compounds (Sari et al., 2012) by enzymatic or non-enzymatic mechanisms. Anthocyanins showed a higher degradation rate in the presence of sugars and their degradation compounds (Amr & Al-Tamimi, 2007; Cavalcanti et al., 2011). The maturation process involves anthocyanin biosynthesis (Table 1), as well as an increase of sucrose and reduced sugars concentrations (data not shown). According to (Lestario et al., 2017), jambolan fruit anthocyanins are mainly composed of delphinidin-3,5-diglucoside, which accounts for 37-48% of TAC, followed by petunidin-3,5-diglucoside (29-33%), malvidin-3,5-diglucoside (19-27%), cyanidin-3,5-diglucoside (3%), delphinidin-3-O-glucoside (2-3%) and peonidin-3,5-diglucoside (1-2%). The same proportion of anthocyanins remains throughout fruit ripening, except for delphinine-3-O-diglucoside and peonidin-3,5-O-diglucoside, which were detected at the green-pink degree of maturity. A similar anthocyanin profile of fully mature jambolan extract was also reported elsewhere (Faria et al., 2011; Sharma et al., 2016).
Different anthocyanins may present different stability against external factors as well. (Türkyılmaz et al., 2022) reported that co-pigmentation with amino acids and aspartic acid, by hydrophobic interactions/hydrogen bond, can slow down the degradation effect of ascorbic acid on anthocyanins in pomegranate and orange juice blend heated at 90-150 °C. According to (Sharma et al., 2016), the storage stability study of jambolan crude extracts showed that malvidin-3,5-diglucoside decreased more than petunidin-3,5-diglucoside, and it was followed by delphinidin-3,5-diglucoside. In addition, anthocyanins present different colors depending on their chemical structure (Cavalcanti et al., 2011). Therefore, the difference in anthocyanin stability, as well as fruit composition, may be responsible for the different degradation rates recorded for powders deriving from different fruit maturity degrees.
3.4 Color stability
Figure 3 presents the color parameters of powders from jambolan fruits at the four degrees of maturity during storage at 25 °C. The L*-value decreased (p ≤ 0.05) during storage time in powders from fruits at the first three maturity degrees (D1 to D3), i.e., samples became darker throughout storage after opening the package, seeing that L*-values reduced by 3 to 6 units. Both packaging materials recorded the same behavior, and no difference was observed between them, except for D4 (p ≤ 0.05). L*-values of powders from fully ripened fruits (D4) were stable, oscillating 3 units for both packings. The minor changes observed might not be significant for practical applications. Similar results were observed for microencapsulated powders of anthocyanins from Hibiscus sabdariffa L. (Idham et al., 2012).
CIE Lab color parameters of jambolan powders from different degrees of maturity during storage in low-density polyethylene (LDPE) and metalized packing. Green (D1); Green-pink (D2); Pink-light purple (D3); Purple-black (D4)
The hue angle decreased with storage time for powders from less mature fruits (D1 and D2) (p ≤ 0.05). This might be attributed to the chlorophyll degradation, as less mature fruits were richer in chlorophyll in comparison to the mature ones. On the other hand, the samples from mature fruits (D3 and D4) showed increased hue angles as storage time increased (p ≤ 0.05). These samples were darker and more purple than the immature ones due to pigment formation, mainly anthocyanins and carotenoids, as well as to chlorophyll decrease (Faria et al., 2011; Hendry & Houghton, 1992; Lestario et al., 2017; Mozetič et al., 2004). The increase of hue values with storage time means that the color moves from violet-purple towards reddish-purple hue. An increase in hue was also observed during storage of foam-mat dried jambolan pulp powder for 150 days (Iasnaia Maria de Carvalho et al., 2017) and SBDs jambolan pulp with egg white-maltodextrin for 40 days (Soares & Pereira, 2020).
Anthocyanins present different colors and stability depending on their chemical structure (Cavalcanti et al., 2011). The main anthocyanins found in mature jambolan fruit, delphinidin-3,5-diglucoside, petunidin-3,5-diglucoside, and malvidin-3,5-diglucoside, present blue/violet hues. As discussed earlier, malvidin and petunidin are less stable than delphinidin, which has a red hue and is a minor anthocyanin found in the fruit (Ananga et al., 2013; Sharma et al., 2016). Therefore, one hypothesis is that powder degradation during storage reflects in less violet/purple hues mainly due to degradation of the major anthocyanins. Hue data showed a good correlation with anthocyanin concentration (0.61≤R2≤0.88) for anthocyanin-rich powders (D4 and D3).
The color difference (Equation 1) increased throughout storage time for samples from four degrees of maturity, ranging from 3 to 7 units on average. This minor color change of powder after several opening of the packages during 90 days of storage, does not impair in loss of the purple attractive color of fruit powder, particularly the ones from the most mature fruits (Figure 1). Concerning the effect of packaging on color stability, the metalized packaging slightly protected the color of all powders.
4 Conclusions
Anthocyanin stability of freeze-dried jambolan fruit powders was significantly affected by storage time, maturity degree, and less affected by package material. The powder of the ripe fruit (D4) was more susceptible to degradation in both packaging types and can be related to the higher hygroscopicity and anthocyanin concentration. Water activity increase was more significant in the transparent packaging (LDPE) type than in the metalized one, with package opening. Overall, the color slightly changed throughout the storage period and the metalized packaging provided stronger protection against color loss and anthocyanin degradation than the transparent one to all jambolan powders. Therefore, to help to preserve the anthocyanins of jambolan powders, it is necessary to store them in high light, oxygen, and moisture-barrier metalized packaging even after opening the package. A period of 90 days retains anthocyanin content by 56% (metalized) to 44% (LDPE) in an opened packing, keeping the pink-purple (D3) and dark-purple (D4) attractive color of powders. Thus, jambolan powders could be indicated as a natural food colorant, with good retention of anthocyanins even after opening the package for 90 days.
Acknowledgements
The authors are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (process 001) and to Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for the financial support (E26/202.858/2015, E-26/010.001764/2015 and E26/010.100966/2018) and postgraduate scholarship (E26/100.606/2014). We are also grateful to Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF) (Laboratório de Tecnologia de Alimentos (LTA) and Laboratório de Fitotecnia (LFIT)) laboratories for the support.
-
Cite as: Mussi, L. P., & Pereira, N. R. (2022). Storage stability of freeze-dried powder of jambolan (Syzygium cumini (L.)) fruits at different degrees of maturity and packages. Brazilian Journal of Food Technology, 25, e2021096. https://doi.org/10.1590/1981-6723.09621
-
Funding: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (process 001); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (E-26/010.001764/2015, E26/100.606/2014, E26/202.858/2015 and E26/010.100966/2018)
References
-
Amr, A., & Al-Tamimi, E. (2007). Stability of the crude extracts of Ranunculus asiaticus anthocyanins and their use as food colourants. International Journal of Food Science & Technology, 42(8), 985-991. http://dx.doi.org/10.1111/j.1365-2621.2006.01334.x
» http://dx.doi.org/10.1111/j.1365-2621.2006.01334.x -
Ananga, A., Georgiev, V., Ochieng, J., Phills, B., & Tsolov, V. (2013). Production of anthocyanins in grape cell cultures: a potential source of raw material for pharmaceutical, food, and cosmetic industries. In B. Sladonja & D. Poljuha (Eds.), The mediterranean genetic code - grapevine and olive (Vol. 32, pp. 137-144). London: InTech. http://dx.doi.org/10.5772/54592
» http://dx.doi.org/10.5772/54592 - Association of Official Analytical Chemists – AOAC. (1998). Official methods of analysis of AOAC International (16th ed.). Arlington: AOAC International.
-
Ayyanar, M., & Subash-Babu, P. (2012). Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pacific Journal of Tropical Biomedicine, 2(3), 240-246. PMid:23569906. http://dx.doi.org/10.1016/S2221-1691(12)60050-1
» http://dx.doi.org/10.1016/S2221-1691(12)60050-1 -
Benherlal, P. S., & Arumughan, C. (2007). Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. Journal of the Science of Food and Agriculture, 87(14), 2560-2569. PMid:20836162. http://dx.doi.org/10.1002/jsfa.2957
» http://dx.doi.org/10.1002/jsfa.2957 -
Bezerra, M., Araujo, A., Santos, K., & Correia, R. (2015). Caprine frozen yoghurt produced with fresh and spray dried jambolan fruit pulp (Eugenia jambolana Lam) and Bifidobacterium animalis subsp. lactis BI-07. Lebensmittel-Wissenschaft + Technologie, 62(2), 1099-1104. http://dx.doi.org/10.1016/j.lwt.2015.01.049
» http://dx.doi.org/10.1016/j.lwt.2015.01.049 -
Borges, K. C., Azevedo, J. C., Medeiros, M. F., & Correia, R. T. P. (2016). Physicochemical characterization and bioactive value of tropical berry pomaces after spouted bed drying. Journal of Food Quality, 39(3), 192-200. http://dx.doi.org/10.1111/jfq.12178
» http://dx.doi.org/10.1111/jfq.12178 -
Braga, M. B., & Rocha, S. C. S. (2015). Spouted bed drying of milk–blackberry pulp: analysis of powder production efficiency and powder characterization. Drying Technology, 33(8), 933-940. http://dx.doi.org/10.1080/07373937.2014.999372
» http://dx.doi.org/10.1080/07373937.2014.999372 -
Cai, Y. Z., & Corke, H. (2000). Production and properties of spray-dried amaranthus betacyanin pigments. Journal of Food Science, 65(7), 1248-1252. http://dx.doi.org/10.1111/j.1365-2621.2000.tb10273.x
» http://dx.doi.org/10.1111/j.1365-2621.2000.tb10273.x -
Cavalcanti, R. N., Santos, D. T., & Meireles, M. A. A. (2011). Non-thermal stabilization mechanisms of anthocyanins in model and food systems: an overview. Food Research International, 44(2), 499-509. http://dx.doi.org/10.1016/j.foodres.2010.12.007
» http://dx.doi.org/10.1016/j.foodres.2010.12.007 -
Cheng, A. W., Xie, H. X., Qi, Y., Liu, C., Guo, X., Sun, J. Y., & Liu, L. N. (2017). Effects of storage time and temperature on polyphenolic content and qualitative characteristics of freeze-dried and spray-dried bayberry powder. LWT, 78, 235-240. http://dx.doi.org/10.1016/j.lwt.2016.12.027
» http://dx.doi.org/10.1016/j.lwt.2016.12.027 - Damodaran, S., Parkin, K. L., & Fennema, O. R. (2007). Fennema’s food chemistry (4th ed.). Boca Raton: CRC Press.
-
Eliasson, L., Labrosse, L., & Ahrné, L. (2017). Effect of drying technique and particle size of bilberry press cake on the extraction efficiency of anthocyanins by pressurized carbon dioxide extraction. Lebensmittel-Wissenschaft + Technologie, 85, 510-516. http://dx.doi.org/10.1016/j.lwt.2017.03.030
» http://dx.doi.org/10.1016/j.lwt.2017.03.030 -
Faria, A. F., Marques, M. C., & Mercadante, A. Z. (2011). Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chemistry, 126(4), 1571-1578. PMid:25213929. http://dx.doi.org/10.1016/j.foodchem.2010.12.007
» http://dx.doi.org/10.1016/j.foodchem.2010.12.007 -
Franks, F. (1998). Freeze-drying of bioproducts: putting principles into practice. European Journal of Pharmaceutics and Biopharmaceutics : Official Journal of Arbeitsgemeinschaft Für Pharmazeutische Verfahrenstechnik e.V., 45(3), 221-229. http://dx.doi.org/10.1016/s0939-6411(98)00004-6
» http://dx.doi.org/10.1016/s0939-6411(98)00004-6 -
Freitas-Sá, D. D. G. C., de Souza, R. C., de Araujo, M. C. P., Borguini, R. G., de Mattos, L. S., Pacheco, S., & Godoy, R. L. O. (2018). Effect of jabuticaba (Myrciaria jaboticaba (Vell) O. Berg) and jamelão (Syzygium cumini (L.) Skeels) peel powders as colorants on color-flavor congruence and acceptability of yogurts. Lwt, 96, 215-221. http://dx.doi.org/10.1016/j.lwt.2018.05.024
» http://dx.doi.org/10.1016/j.lwt.2018.05.024 -
Fujita, A., Borges, K., Correia, R., Franco, B. D. G. D. M., & Genovese, M. I. (2013). Impact of spouted bed drying on bioactive compounds, antimicrobial and antioxidant activities of commercial frozen pulp of camu-camu (Myrciaria dubia Mc. Vaugh). Food Research International, 54(1), 495-500. http://dx.doi.org/10.1016/j.foodres.2013.07.025
» http://dx.doi.org/10.1016/j.foodres.2013.07.025 -
Gradinaru, G., Biliaderis, C. G., Kallithraka, S., Kefalas, P., & Garcia-Viguera, C. (2003). Thermal stability of Hibiscus sabdariffa L. anthocyanins in solution and in solid state: effects of copigmentation and glass transition. Food Chemistry, 83(3), 423-436. http://dx.doi.org/10.1016/S0308-8146(03)00125-0
» http://dx.doi.org/10.1016/S0308-8146(03)00125-0 -
Grover, J. K., Vats, V., & Rathi, S. S. (2000). Anti-hyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. Journal of Ethnopharmacology, 73(3), 461-470. PMid:11091000. http://dx.doi.org/10.1016/S0378-8741(00)00319-6
» http://dx.doi.org/10.1016/S0378-8741(00)00319-6 -
Hazarika, U., & Gosztola, B. (2020). Lyophilization and its effects on the essential oil content and composition of herbs and spices - a review. Acta Scientiarum Polonorum. Technologia Alimentaria, 19(4), 467-473. PMid:33179486. http://dx.doi.org/10.17306/J.AFS.2020.0853
» http://dx.doi.org/10.17306/J.AFS.2020.0853 - Hendry, G. A. F., & Houghton, J. D. (1992). Natural food colorants USA: Blackie Academic.
-
Henríquez, C., Córdova, A., Lutz, M., & Saavedra, J. (2013). Storage stability test of apple peel powder using two packaging materials: high-density polyethylene and metalized films of high barrier. Industrial Crops and Products, 45, 121-127. http://dx.doi.org/10.1016/j.indcrop.2012.11.032
» http://dx.doi.org/10.1016/j.indcrop.2012.11.032 -
Iasnaia Maria de Carvalho, T., Nogueira, T. Y. K., Mauro, M. A., Gómez-Alonso, S., Gomes, E., Da-Silva, R., Hermosín-Gutiérrez, I., & Lago-Vanzela, E. S. (2017). Dehydration of jambolan [Syzygium cumini (L. )] juice during foam mat drying: quantitative and qualitative changes of the phenolic compounds. Food Research International, 102, 32-42. PMid:29195954. http://dx.doi.org/10.1016/j.foodres.2017.09.068
» http://dx.doi.org/10.1016/j.foodres.2017.09.068 -
Idham, Z., Muhamad, I. I., & Sarmidi, M. R. (2012). Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from Hibiscus sabdariffa L. Journal of Food Process Engineering, 35(4), 522-542. http://dx.doi.org/10.1111/j.1745-4530.2010.00605.x
» http://dx.doi.org/10.1111/j.1745-4530.2010.00605.x -
Konica Minolta. (1998). Precise color communication: color control from feeling to instrumentation Retrieved April 12, 2018, from https://www.konicaminolta.com/instruments/knowledge/color/pdf/color_communication.pdf
» https://www.konicaminolta.com/instruments/knowledge/color/pdf/color_communication.pdf -
Lee, J., Durst, R. W., & Wrolstad, R. E. (2005). Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. Journal of AOAC International, 88(5), 1269-1278. PMid:16385975. http://dx.doi.org/10.1093/jaoac/88.5.1269
» http://dx.doi.org/10.1093/jaoac/88.5.1269 -
Lestario, L. N., Howard, L. R., Brownmiller, C., Stebbins, N. B., Liyanage, R., & Lay, J. O. (2017). Changes in polyphenolics during maturation of Java plum (Syzygium cumini Lam.). Food Research International, 100(Pt 3), 385-391. PMid:28964361. http://dx.doi.org/10.1016/j.foodres.2017.04.023
» http://dx.doi.org/10.1016/j.foodres.2017.04.023 -
Longo, L., Scardino, A., Vasapollo, G., & Blando, F. (2007). Anthocyanins from Eugenia myrtifolia Sims. Innovative Food Science & Emerging Technologies, 8(3), 329-332. http://dx.doi.org/10.1016/j.ifset.2007.03.023
» http://dx.doi.org/10.1016/j.ifset.2007.03.023 -
Maltini, E., Torreggiani, D., Venir, E., & Bertolo, G. (2003). Water activity and the preservation of plant foods. Food Chemistry, 82(1), 79-86. http://dx.doi.org/10.1016/S0308-8146(02)00581-2
» http://dx.doi.org/10.1016/S0308-8146(02)00581-2 -
Menezes, M. L., Ströher, A. P., Pereira, N. C., & De Barros, S. T. D. (2013). Análise da cinética e ajustes de modelos matemáticos aos dados de secagem do bagaço do maracujá-amarelo. Engevista, 15(2), 176-186. http://dx.doi.org/10.22409/engevista.v15i2.443
» http://dx.doi.org/10.22409/engevista.v15i2.443 -
Mozetič, B., Trebše, P., Simčič, M., & Hribar, J. (2004). Changes of anthocyanins and hydroxycinnamic acids affecting the skin colour during maturation of sweet cherries (Prunus avium L.). Lebensmittel-Wissenschaft + Technologie, 37(1), 123-128. http://dx.doi.org/10.1016/S0023-6438(03)00143-9
» http://dx.doi.org/10.1016/S0023-6438(03)00143-9 - Mussi, L. P. (2018). Aproveitamento do fruto de jambolão e resíduo da polpa: efeito da maturação na secagem, características físicas, químicas, bioativas e estabilidade dos produtos secos. Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes.
-
Mussi, L. P., Guimarães, A. O., Ferreira, K. S., & Pereira, N. R. (2015). Spouted bed drying of jambolão (Syzygium cumini) residue: drying kinetics and effect on the antioxidant activity, anthocyanins and nutrients contents. Lebensmittel-Wissenschaft + Technologie, 61(1), 80-88. http://dx.doi.org/10.1016/j.lwt.2014.11.040
» http://dx.doi.org/10.1016/j.lwt.2014.11.040 -
Nemzer, B., Vargas, L., Xia, X., Sintara, M., & Feng, H. (2018). Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chemistry, 262, 242-250. PMid:29751916. http://dx.doi.org/10.1016/j.foodchem.2018.04.047
» http://dx.doi.org/10.1016/j.foodchem.2018.04.047 -
Patel, P. R., & Rao, T. V. R. (2014). Growth and ripening in black plum [Syzygium cumini (L.) Skeels]. International Journal of Fruit Science, 14(2), 147-156. http://dx.doi.org/10.1080/15538362.2013.817842
» http://dx.doi.org/10.1080/15538362.2013.817842 -
Santiago, M. C. P. A., Gouvêa, A. C. M. S., Peixoto, F. M., Borguini, R. G., Godoy, R. L., Pacheco, S., Nascimento, L. S. M., & Nogueira, R. I. (2016). Characterization of jamelão (Syzygium cumini (L.) Skeels) fruit peel powder for use as natural colorant. Fruits, 71(1), 3-8. http://dx.doi.org/10.1051/fruits/2015041
» http://dx.doi.org/10.1051/fruits/2015041 -
Santos, C. A., Almeida, F. A., Quecán, B. X. V., Pereira, P. A. P., Gandra, K. M. B., Cunha, L. R., & Pinto, U. M. (2020). Bioactive properties of Syzygium cumini (L.) skeels pulp and seed phenolic extracts. Frontiers in Microbiology, 11, 990. PMid:32528438. http://dx.doi.org/10.3389/fmicb.2020.00990
» http://dx.doi.org/10.3389/fmicb.2020.00990 -
Sari, P., Wijaya, C. H., Sajuthi, D., & Supratman, U. (2012). Colour properties, stability, and free radical scavenging activity of jambolan (Syzygium cumini) fruit anthocyanins in a beverage model system: natural and copigmented anthocyanins. Food Chemistry, 132(4), 1908-1914. http://dx.doi.org/10.1016/j.foodchem.2011.12.025
» http://dx.doi.org/10.1016/j.foodchem.2011.12.025 -
Sharma, R. J., Gupta, R. C., Singh, S., Bansal, A. K., & Singh, I. P. (2016). Stability of anthocyanins- and anthocyanidins-enriched extracts, and formulations of fruit pulp of Eugenia jambolana (‘jamun’). Food Chemistry, 190, 808-817. PMid:26213042. http://dx.doi.org/10.1016/j.foodchem.2015.06.029
» http://dx.doi.org/10.1016/j.foodchem.2015.06.029 -
Sinela, A., Rawat, N., Mertz, C., Achir, N., Fulcrand, H., & Dornier, M. (2017). Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chemistry, 214, 234-241. PMid:27507471. http://dx.doi.org/10.1016/j.foodchem.2016.07.071
» http://dx.doi.org/10.1016/j.foodchem.2016.07.071 -
Singh, J. P., Kaur, A., Singh, N., Nim, L., Shevkani, K., Kaur, H., & Arora, D. S. (2016). In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. Lebensmittel-Wissenschaft + Technologie, 65, 1025-1030. http://dx.doi.org/10.1016/j.lwt.2015.09.038
» http://dx.doi.org/10.1016/j.lwt.2015.09.038 -
Skrede, G., Wrolstad, R. E., & Durst, R. W. (2000). Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). Journal of Food Science, 65(2), 357-364. http://dx.doi.org/10.1111/j.1365-2621.2000.tb16007.x
» http://dx.doi.org/10.1111/j.1365-2621.2000.tb16007.x -
Soares, A. C., & Pereira, N. R. (2020). Secagem da polpa de jambolão (Syzygium cumini) em secador de leito de jorro: efeito da clara de ovo como agente carreador de secagem na qualidade do produtoy. Brazilian Journal of Food Technology, 23, e2019075. http://dx.doi.org/10.1590/1981-6723.07519
» http://dx.doi.org/10.1590/1981-6723.07519 -
Sturm, K., Koron, D., & Stampar, F. (2003). The composition of fruit of different strawberry varieties depending on maturity stage. Food Chemistry, 83(3), 417-422. http://dx.doi.org/10.1016/S0308-8146(03)00124-9
» http://dx.doi.org/10.1016/S0308-8146(03)00124-9 -
Tavares, I. M. C., Sumere, B. R., Gómez-Alonso, S., Gomes, E., Hermosín-Gutiérrez, I., Da-Silva, R., & Lago-Vanzela, E. S. (2020). Storage stability of the phenolic compounds, color and antioxidant activity of jambolan juice powder obtained by foam mat drying. Food Research International, 128, 108750. PMid:31955732. http://dx.doi.org/10.1016/j.foodres.2019.108750
» http://dx.doi.org/10.1016/j.foodres.2019.108750 -
Tonon, R. V., Brabet, C., & Hubinger, M. D. (2008). Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. Journal of Food Engineering, 88(3), 411-418. http://dx.doi.org/10.1016/j.jfoodeng.2008.02.029
» http://dx.doi.org/10.1016/j.jfoodeng.2008.02.029 -
Tonon, R. V., Brabet, C., & Hubinger, M. D. (2010). Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Research International, 43(3), 907-914. http://dx.doi.org/10.1016/j.foodres.2009.12.013
» http://dx.doi.org/10.1016/j.foodres.2009.12.013 -
Türkyılmaz, M., Hamzaoğlu, F., Ünal, H., & Özkan, M. (2022). Influence of amino acid addition on the thermal stability of anthocyanins in pomegranate (Punica granatum L., cv. Hicaznar) and orange (Citrus sinensis L. Osbeck, cv. Valencia) juice blend. Food Chemistry, 370, 131061. PMid:34547556. http://dx.doi.org/10.1016/j.foodchem.2021.131061
» http://dx.doi.org/10.1016/j.foodchem.2021.131061 -
Udomkun, P., Nagle, M., Argyropoulos, D., Mahayothee, B., Latif, S., & Müller, J. (2016). Compositional and functional dynamics of dried papaya as affected by storage time and packaging material. Food Chemistry, 196, 712-719. PMid:26593545. http://dx.doi.org/10.1016/j.foodchem.2015.09.103
» http://dx.doi.org/10.1016/j.foodchem.2015.09.103 -
Usenik, V., Štampar, F., & Veberič, R. (2009). Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chemistry, 114(2), 529-534. http://dx.doi.org/10.1016/j.foodchem.2008.09.083
» http://dx.doi.org/10.1016/j.foodchem.2008.09.083 -
Veigas, J., Narayan, M., Laxman, P., & Neelwarne, B. (2007). Chemical nature, stability and bioefficacies of anthocyanins from fruit peel of syzygium cumini skeels. Food Chemistry, 105(2), 619-627. http://dx.doi.org/10.1016/j.foodchem.2007.04.022
» http://dx.doi.org/10.1016/j.foodchem.2007.04.022 -
Vikrant, V., Grover, J. K., Tandon, N., Rathi, S. S., & Gupta, N. (2001). Treatment with extracts of Momordica charantia and Eugenia jambolana prevents hyperglycemia and hyperinsulinemia in fructose fed rats. Journal of Ethnopharmacology, 76(2), 139-143. PMid:11390126. http://dx.doi.org/10.1016/S0378-8741(01)00218-5
» http://dx.doi.org/10.1016/S0378-8741(01)00218-5 -
Waghmare, R. B., Perumal, A. B., Moses, J. A., & Anandharamakrishnan, C. (2021). Recent developments in freeze drying of foods. In K. Knoerzer & K. Muthukumarappan (Eds.), Innovative food processing technologies: a comprehensive review (pp. 82-99). https://doi.org/10.1016/B978-0-12-815781-7.00017-2
» https://doi.org/10.1016/B978-0-12-815781-7.00017-2 -
Wrolstad, R. E. (2004). Anthocyanin pigments - Bioactivity and coloring properties. Journal of Food Science, 69(5), C419-C421. http://dx.doi.org/10.1111/j.1365-2621.2004.tb10709.x
» http://dx.doi.org/10.1111/j.1365-2621.2004.tb10709.x -
Zorić, Z., Pedisić, S., Kovačević, D. B., Ježek, D., & Dragović-Uzelac, V. (2016). Impact of packaging material and storage conditions on polyphenol stability, colour and sensory characteristics of freeze-dried sour cherry (prunus cerasus var. Marasca). Journal of Food Science and Technology, 53(2), 1247-1258. PMid:27162405. http://dx.doi.org/10.1007/s13197-015-2097-4
» http://dx.doi.org/10.1007/s13197-015-2097-4
Edited by
-
Associate Editor: Rosinelson da Silva Pena.
Publication Dates
-
Publication in this collection
09 May 2022 -
Date of issue
2022
History
-
Received
02 June 2021 -
Accepted
21 Feb 2022

 Storage stability of freeze-dried powder of jambolan (Syzygium cumini (L.)) fruits at different degrees of maturity and packages
Storage stability of freeze-dried powder of jambolan (Syzygium cumini (L.)) fruits at different degrees of maturity and packages