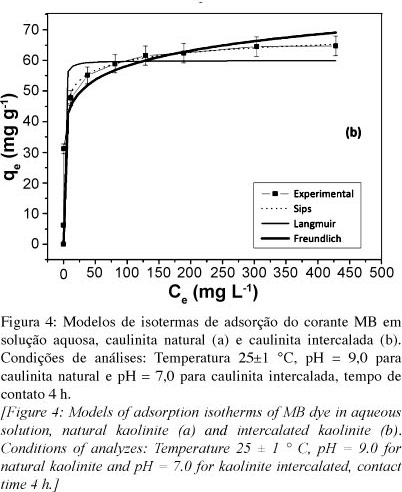
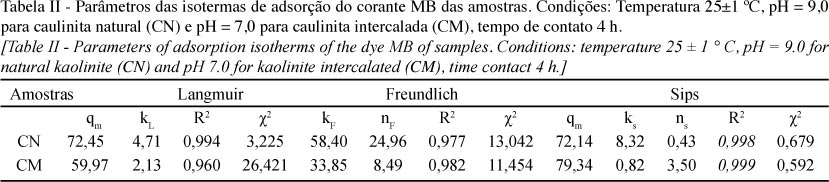
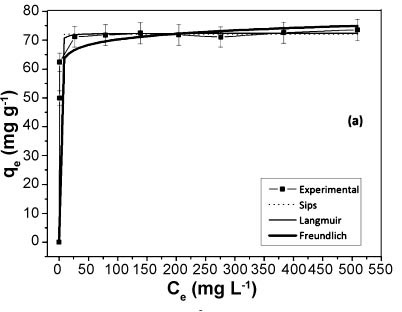
The present study aimed to evaluate the adsorption capacity of methylene blue dye in aqueous solutions by natural kaolinite from the region of Bom Jardim de Goiás, GO, Brazil, and its form intercalated with the organic compound potassium acetate. The natural sample and intercalated were characterized by diffraction and X-ray fluorescence, scanning electron microscopy and infrared. The intercalation process resulted in an increase in the basal spacing (d001) in the clay mineral structure, which may be verified by X-ray diffraction. The capacity of samples to remove methylene blue from aqueous solutions was investigated by the batch method and the data were calculated by the non-linear method using model the Langmuir, Freundlich and Sips. The model that best fitted the experimental data was the Sips for both samples. For kinetic studies have been tested three models, pseudo-first order, pseudo-second order, and Avrami. For both samples the Avrami model was the best fit to the experimental data. The maximum adsorption capacity reached by the intercalated sample was 79,34 mg.g-1 and natural was of 72,14 mg.g-1. These values show that natural and intercalated kaolinite may be considered good adsorbents to the removal of methylene blue dye in aqueous medium.
kaolinite; intercalation; potassium acetate; adsorption; methylene blue