Abstract
OBJECTIVES: Acute retinal necrosis is a rapidly progressive and devastating viral retinitis caused by the herpesvirus family. Systemic acyclovir is the treatment of choice; however, the progression of retinal lesions ceases approximately 2 days after treatment initiation. An intravitreal injection of acyclovir may be used an adjuvant therapy during the first 2 days of treatment when systemically administered acyclovir has not reached therapeutic levels in the retina. The aims of this study were to determine the pharmacokinetic profile of acyclovir in the rabbit vitreous after intravitreal injection and the functional effects of acyclovir in the rabbit retina. METHODS: Acyclovir (Acyclovir; Bedford Laboratories, Bedford, OH, USA) 1 mg in 0.1 mL was injected into the right eye vitreous of 32 New Zealand white rabbits, and 0.1 mL sterile saline solution was injected into the left eye as a control. The animals were sacrificed after 2, 9, 14, or 28 days. The eyes were enucleated, and the vitreous was removed. The half-life of acyclovir was determined using high-performance liquid chromatography. Electroretinograms were recorded on days 2, 9, 14, and 28 in the eight animals that were sacrificed 28 days after injection according to a modified protocol of the International Society for Clinical Electrophysiology of Vision. RESULTS: Acyclovir rapidly decayed in the vitreous within the first two days after treatment and remained at low levels from day 9 onward. The eyes that were injected with acyclovir did not present any electroretinographic changes compared with the control eyes. CONCLUSIONS: The vitreous half-life of acyclovir is short, and the electrophysiological findings suggest that the intravitreal delivery of 1 mg acyclovir is safe and well tolerated by the rabbit retina.
Acyclovir; Pharmacokinetics; Electroretinography; Drug Toxicity; Retina
BASIC RESEARCH
Vitreous pharmacokinetics and electroretinographic findings after intravitreal injection of acyclovir in rabbits
Francisco Max DamicoI; Mariana Ramos ScolariI; Gabriela Lourençon IoshimotoII; Beatriz Sayuri TakahashiI; Armando da Silva Cunha Jr.III; Sílvia Ligório FialhoIV; Daniela Maria BonciII; Fabio GasparinI; Dora Fix VenturaII
IFaculdade de Medicina da Universidade de São Paulo, São Paulo/SP, Brazil
IIUniversidade de São Paulo, Instituto de Psicologia, São Paulo/SP, Brazil
IIIUniversidade Federal de Minas Gerais, Faculdade de Farmácia, Belo Horizonte/MG, Brazil
IVFundação Ezequiel Dias, Belo Horizonte/MG, Brazil
ABSTRACT
OBJECTIVES: Acute retinal necrosis is a rapidly progressive and devastating viral retinitis caused by the herpesvirus family. Systemic acyclovir is the treatment of choice; however, the progression of retinal lesions ceases approximately 2 days after treatment initiation. An intravitreal injection of acyclovir may be used an adjuvant therapy during the first 2 days of treatment when systemically administered acyclovir has not reached therapeutic levels in the retina. The aims of this study were to determine the pharmacokinetic profile of acyclovir in the rabbit vitreous after intravitreal injection and the functional effects of acyclovir in the rabbit retina.
METHODS: Acyclovir (Acyclovir; Bedford Laboratories, Bedford, OH, USA) 1 mg in 0.1 mL was injected into the right eye vitreous of 32 New Zealand white rabbits, and 0.1 mL sterile saline solution was injected into the left eye as a control. The animals were sacrificed after 2, 9, 14, or 28 days. The eyes were enucleated, and the vitreous was removed. The half-life of acyclovir was determined using high-performance liquid chromatography. Electroretinograms were recorded on days 2, 9, 14, and 28 in the eight animals that were sacrificed 28 days after injection according to a modified protocol of the International Society for Clinical Electrophysiology of Vision.
RESULTS: Acyclovir rapidly decayed in the vitreous within the first two days after treatment and remained at low levels from day 9 onward. The eyes that were injected with acyclovir did not present any electroretinographic changes compared with the control eyes.
CONCLUSIONS: The vitreous half-life of acyclovir is short, and the electrophysiological findings suggest that the intravitreal delivery of 1 mg acyclovir is safe and well tolerated by the rabbit retina.
Keywords: Acyclovir; Pharmacokinetics; Electroretinography; Drug Toxicity; Retina.
INTRODUCTION
Acute retinal necrosis (ARN) is a rapidly progressive viral retinitis that is caused by members of the herpesvirus family and has an unfavorable visual prognosis (1,2). Prompt recognition of ARN is important, and patients who are treated early with high doses of intravenous acyclovir have a better visual outcome and a lower incidence of retinal detachment (3).
Acyclovir is a potent systemic antiviral that has been the drug of choice for the treatment of ARN because of its selectivity against the herpes simplex virus and the varicella-zoster virus (4,5). The goal of treatment is to stop the progression of retinal lesions. Clinically, the progression of existing retinal lesions usually ceases approximately 2 days after the initiation of treatment with intravenous acyclovir, and visible regression may be observed after approximately 4 days (1). Complete regression occurs after an average of 32.5 days when the retina may have already been irreversibly damaged (1).
To penetrate the eye, drugs must overcome the bloodocular barrier, which greatly decreases their bioavailability to the retina (6). Intravitreal injections bypass the bloodretinal barrier and thus provide immediate drug delivery at therapeutic levels directly to the target site.
There is evidence that the time between diagnosis and the initiation of treatment may be a prognostic factor and that the reduction of this interval may improve the outcome of ARN (7). We postulate that the intravitreal injection of acyclovir as soon as the diagnosis of ARN has been established may be a potential adjuvant therapy because it may help to control this type of aggressive retinitis within the crucial first 48 hours, when systemic acyclovir has not reached therapeutic levels in the retina.
In this study, we investigated the pharmacokinetic profile and functional effects of acyclovir in the rabbit vitreous and the retina, respectively, at different time points after an intravitreal injection.
METHODS
Animals
In total, 32 New Zealand white rabbits (weight, 2.0-2.5 kg) were included in this study. The animals were treated according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The experiments were approved by the Research Ethics Committee of the Universidade de São Paulo, Faculdade de Medicina and by the Committee for Ethics in Animal Research of the Instituto de Psicologia at the Universidade de São Paulo (São Paulo, Brazil).
The animals were housed in individual cages under a 12/ 12-hour light-dark cycle with free access to water and food. Before every procedure (intravitreal injection, ERG, and sacrifice), the animals were anesthetized with an intramuscular injection of 50 mg/kg ketamine hydrochloride (Ketamina; Agener, São Paulo, Brazil) and 6.7 mg/kg xylazine hydrochloride (Calmiun; Agener, São Paulo, Brazil). The pupils were dilated with topical 0.5% tropicamide (Mydriacyl; Alcon, São Paulo, Brazil), and the eyes were anesthetized with 0.5% proxymetacaine hydrochloride (Anestalcon; Alcon, São Paulo, Brazil). A slit-lamp examination and indirect fundus ophthalmoscopy were performed in all of the eyes prior to the intravitreal injections and after 2, 9, 14, and 28 days to detect signs of inflammation or infection. All of the animals were sacrificed with an intravenous injection of 70 mg/kg sodium pentobarbital (Euthanyle; Brouwer, Buenos Ayres, Argentina) under deep anesthesia.
Acyclovir preparation
The original solution of acyclovir (Acyclovir; Bedford Laboratories, Bedford, OH) was diluted with sterile saline solution (saline) to reach a concentration of 10 mg/mL for injection, and 0.1 mL (1 mg) was injected directly into the vitreous.
Intravitreal injection
Before the injections, anterior chamber paracentesis (0.1 mL of the aqueous humor) was performed with a 27 gauge needle to prevent an increase in intraocular pressure and minimize drug reflux. The intravitreal injections were performed using a 30-gauge needle that was attached to a 1mL tuberculin syringe, which was inserted approximately 3 mm posterior to the limbus, and 0.1 mL acyclovir was slowly injected directly into the vitreous. The right eye of each rabbit was injected with the acyclovir solution and the left eye was injected with saline as a control.
High-performance liquid chromatography for vitreous half-life determination
To determine the half-life of acyclovir in the vitreous, the animals were divided into four groups of eight animals each. All the animals received an intravitreal injection of 1 mg/0.1 mL acyclovir in the right eye and 0.1 mL saline in the left eye. The animals were sacrificed on days 2, 9, 14, and 28 after intravitreal injection. The eyes were enucleated, the anterior segment and lens were discarded, and the vitreous body was removed and frozen at -18ºC for a high-performance liquid chromatography (HPLC) assay. Peripheral blood was drawn at the time of sacrifice to determine the systemic concentration of acyclovir after intravitreal injection. The samples were frozen at -18ºC for HPLC.
HPLC for the determination of vitreous and systemic acyclovir levels has been previously described (8-11). Briefly, the chromatographic system consisted of a Merck-Hitachi LaChrom Elite apparatus that was equipped with an autosampler with a sample loop of 100 µL (model L2200, Merck-Hitachi, Germany), a pump with a constant flow rate of 1.4 mL/min (model L-2130, Merck-Hitachi, Germany), and a diode array detector with a wavelength of 215 nm (model L-2450; Merck-Hitachi, Germany). Separation chromatography was performed using an Ace 5 C18 column (250×4.6 mm id, Advanced Chromatography Technologies, Scotland) that was maintained at 50ºC (column oven model L-2300, Merck-Hitachi, Germany). A mixture of acetonitrile (Merck, Darmstadt, Germany) and 40 mM phosphoric acid buffer (Omega, Belo Horizonte, Brazil) at pH 3.0 (32:68 v/v) was used as the mobile phase. Under these experimental conditions, the retention time was 14.0 min.
Sample treatment: Frozen vitreous samples were thawed at ambient temperature. After brief mixing, 500 µL of the mobile phase was added to 500-µL aliquots of the thawed vitreous and blood samples. After mixing for 1.0 min, the samples were filtered (Durapore, 0.2 µm, Millipore) and 100 µL was injected into the column. To reduce experimental variability, all the samples were measured together in the same assay.
A standard stock solution was prepared in methanol that contained 1 mg/mL acyclovir. This solution was added to drug-free rabbit vitreous and blood to prepare six non-zero concentrations in the range of 0.2515.0 µg/mL acyclovir (0.25, 0.5, 2.0, 5.0, 10.0, and 15.0 µg/mL).
The ocular pharmacokinetic model was developed using previously described studies as a reference (12,13). All the data were fit with a single exponent according to Equation 1, and the estimated half-time (t1/2) of acyclovir elimination was calculated with Equation 2.
C(t)= Coexp(-kt)
t1/2 = 0.693/k
where C (µg/mL) and C0 (µg/mL) represent respectively the acyclovir concentration at any time and at t0, t (day) is the time after injection, and k (day-1) represents a rate constant.
Electroretinography
To evaluate the effect of acyclovir on retinal function, electroretinograms (ERGs) were recorded in the 8 animals that were sacrificed 28 days after injection. ERG recordings were obtained on days 2, 9, 14, and 28 after intravitreal injection.
The ERG protocol was based on the international standard for electroretinography from the International Society for Clinical Electrophysiology of Vision (ISCEV) (14). The animals were dark-adapted for 1 h and anesthetized with a mixture of ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (6.7 mg/kg), and the pupils were dilated 15 min before ERG using a topical application of 0.5% tropicamide (Mydriacyl; Alcon, São Paulo, Brazil). Immediately before the ERG recordings, the eyes were topically anesthetized with 0.5% proxymetacaine hydrochloride (Anestalcon; Alcon, São Paulo, Brazil).
The ERG responses from both eyes were recorded using a corneal bipolar contact lens electrode (GoldLens; Doran Instruments Inc., Littleton, MA). Reference electrodes were placed in the skin near the lateral canthus of the eyes, and a ground electrode (model E5; Grass Technologies, West Warwick, RI) was placed on the ear.
Light stimulation was provided by a Ganzfeld LED stimulator (Q450 SC Roland-Consult, Germany) and was controlled by a computerized system (RetiPort, Roland Consult, Germany).
ERGs were recorded according to a modified ISCEV protocol (14,15):
1) Dark-adapted ERG: Five stimulus intensities were tested after dark adaptation: a) 10 flashes were presented at 0.0003 cd.s/m2 with 5-s interflash intervals; b) 6 flashes were presented at 0.003 cd.s/m2 with 5-s interflash intervals; c) after 20 s of dark adaptation, 6 flashes were presented at 0.03 cd.s/m2 with an interflash interval of 10 s; d) after 1 min of dark adaptation, 6 flashes were presented at 0.3 cd.s/m2 with an interflash interval of 10 s; and e) after 1 min of dark adaptation, 3 flashes were presented at 3.0 cd.s/m2 with an interflash interval of 15 s.
2) Light-adapted ERG: A 2-min light adaptation was conducted with a background of 25 cd.s/m2 (white light) using an average of 6 flashes at 3.0 cd.s/m2 with an interflash interval of 5 s.
ERG data analysis: The a-and b-wave amplitudes and the implicit times were measured. The a-wave amplitude was measured from the baseline to the minimum amplitude after light stimulus onset. The a-wave time to peak or implicit time was measured from flash onset to the a-wave peak. The b-wave amplitude was measured from the awave through the b-wave peak amplitude. Similarly, the bwave implicit time corresponded to the time of occurrence of its peak amplitude (14).
The correlation between the dark-adapted b-wave amplitude and the stimulus luminance was modeled using the Naka-Rushton function. The following three parameters were obtained: the b-wave saturating amplitude (Vmax); the dark-adapted sensitivity, which was defined as the intensity necessary for a response amplitude of 50% of the Vmax (k); and the exponential of the Naka-Rushton equation (n), which is related to the slope in the linear phase of the sigmoid function and represents the homogeneity of the retinal sensitivity.
Analysis
The statistical analysis was performed using Statistica software (StatSoft v6.0, Inc., Tulsa, OK, USA,). The assessment of the significant differences among the groups was performed using the repeated-measures ANOVA test, which takes into account the strength of the correlations and provides information regarding the variables. The Fisher's Least Significant Differences Test was used to determine the differences between the group means in the repeated-measures ANOVA tests.
RESULTS
Clinical findings
No cataracts, anterior chamber cells, vitreous cells, retinal lesions, or cases of endophthalmitis were detected in the eyes that were injected with acyclovir or saline at any time point during the study period.
Acyclovir pharmacokinetics
The HPLC method was validated, and a calibration curve was obtained. The HPLC sensitivity was 0.1 µg/mL. The vitreous concentrations of acyclovir over 28 days are presented in Figure 1. The mean intravitreal concentrations of acyclovir (µg/mL) were 0.28, 0.09, 0.06, and 0.08 on days 2, 9, 14, and 28 after injection, respectively. The half-life of acyclovir in the rabbit vitreous could not be estimated because the drug was quickly eliminated and only a small amount was detected on the second day after injection.
Acyclovir was not detected in the peripheral blood of any animal that was tested.
ERG
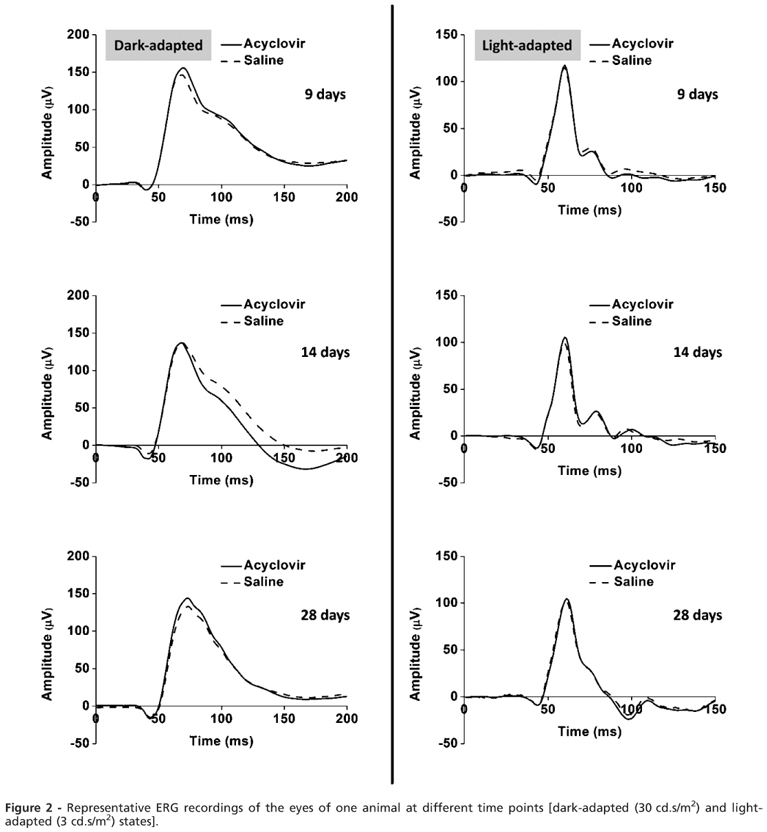
The representative dark-and light-adapted ERG recordings of one animal at different time points are shown in Figure 2. The eyes that were injected with acyclovir were compared with the eyes that were injected with saline. In the dark-and light-adapted states, no significant differences were found between the means of the a-and b-wave amplitudes and the implicit times for any time point or light intensity. The data from the dark-adapted ERG recordings are shown in Table 1.
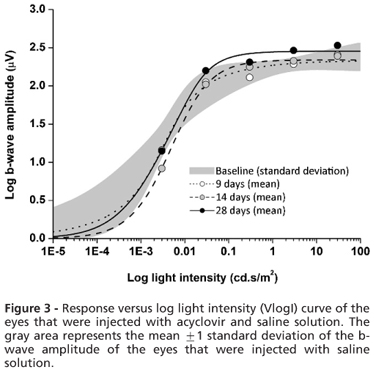
The dark-and light-adapted a-wave and b-wave mean amplitudes were plotted against the log light intensity (VlogI) using the Naka-Rushton equation. The curves at each time point were compared to the mean baseline curve. No significant differences were found for the a-wave or b-wave amplitudes on days 9, 14, and 28 compared with the baseline. The data for the b-wave amplitudes are presented in Figure 3.
The Vmax, k, and n values were calculated for the b-wave amplitudes, and no significant differences were found between the eyes that were injected with acyclovir and saline. The b-wave Vmax, k, and n values are presented in Table 2.
The b-wave to a-wave correlations for the dark-adapted state (30 cd.s/m2) are shown in Figure 4. No significant differences were found among the different time points.
Oscillatory potentials could not be detected in any of the eyes that were studied.
DISCUSSION
Our ERG results suggest that intravitreal injection of acyclovir 1 mg in 0.1 mL does not cause functional changes in the retina of New Zealand white rabbits according to binocular indirect ophthalmoscopy and ERG during a 28-day follow-up period.
This is the first report of experimental data on the retinal effects of 1 mg acyclovir injected directly into the vitreous. The injected dose was estimated based on the dose that was used for the intravenous injections and on a dose-escalating study (Damico FM et al., unpublished), which demonstrated that 1 mg acyclovir injected into the vitreous does not cause morphological changes in the rabbit retina, according to light microscopy.
ERG is a useful tool that is commonly employed to monitor retinal toxicity (16). In addition to the determination of a-and b-wave amplitudes and implicit times in lightand dark-adapted states, many correlations and other parameters can be analyzed. The analysis of the correlation between b-wave amplitude and light intensity at different time points offers an estimate of the functional integrity of the retina. Different ERG components are related to different retinal structures. The cornea negative a-wave reflects the function of the photoreceptors; the b-wave reflects the function of bipolar and Mueller cells; and the oscillatory potentials reflect the activity of the inner retina (17). Moreover, the b-wave to a-wave amplitude ratio is an index of post-receptoral function that represents the effect of a given stimulus in the inner and outer retina (18).
In this study, ERG recordings were obtained at baseline and at 3 different time points after intravitreal injection of acyclovir into the study eye and saline into the contralateral eye. ERG parameters are influenced by many factors, such as pupil size, dark adaptation time, electrode, stimulus intensity, age, breed, anesthetic drugs, and body temperature (19,20); however, it is difficult to control for these factors. Our results presented low variability (Table 1), which reflects the high quality of the ERG technique.
There were no significant differences between the a-and b-wave amplitude values and the implicit time values, which suggests that the inner and outer retina were not functionally impaired by the dose of acyclovir that was used in this study. In addition, the b-wave to a-wave ratios were not different between the eyes that were injected with acyclovir and saline, which suggests that the 1-mg dose of acyclovir does not cause the deterioration of retinal function. However, full-field ERG is a mass electrical response of the retina to light stimulation, and acyclovir may cause focal damage to the retina that may not be detected by testing. Intravitreal injections of bevacizumab do not induce signs of toxicity in the rabbit retina according to ERG (21,22). However, inflammatory cells and signs of apoptosis in the photoreceptors were observed using transmission electron microscopy in these studies. Similar findings were obtained for a high-dose intravitreal injection of moxifloxacin (23).
Experimental studies in the 1980s evaluated the retinal toxicity of acyclovir after intravitreal injection (24-26) and demonstrated that doses of up to 450 mg/0.1 mL do not cause retinal toxicity; however, the same animals were not used as controls. In contrast, in the present study the contralateral eye of the same animal was used as control. The use of different animals as controls may not be adequate because of the high inter-animal variability in ERG parameters. In addition, the ERG methods and data were not well described in the previous studies, which makes comparisons with our results difficult.
The pharmacokinetic study revealed that systemic acyclovir absorption after intravitreal injection may not be significant because the drug was not detected in the peripheral blood of any animal.
This is the first report on the vitreous half-life of acyclovir. HPLC indicated a rapid decay in the concentration of acyclovir in the vitreous during the first two days after intravitreal injection, after which the acyclovir was maintained at low levels from day 9 on. Acyclovir is a Biopharmaceutics Classification System Class III compound and is highly water-soluble with low permeability and a low molecular mass. In addition, the systemic half-life of acyclovir after oral intake has been observed to vary from 2.5 to 3 h (27,28). In rabbits, the systemic half-life is even lower, varying from 1 to 2 h (29). These results suggest that acyclovir may present a short half-life in the vitreous, which may explain the rapid clearance from the vitreous that was observed in this study. However, acyclovir would not be detected if it were bound to serum proteins (30). This hypothesis was not tested in the present study. In this study, the determination of the vitreous half-life of acyclovir was not possible because of the intervals that were chosen for sample collection. However, our results suggest that the vitreous half-life of acyclovir may be very short, such as less than 48 h.
This study has several limitations. The relatively low number of animals that underwent ERG assessment (eight animals) may mask differences between the eyes that were injected with acyclovir and saline. However, our results had low variability, which suggests that they are reliable. In addition, acyclovir is highly soluble in water and vitreous clearance is likely to be very short. ERG was recorded at baseline and on day 9, when a small amount of acyclovir was present in the vitreous. Thus, acyclovir may have caused functional retinal damage within the first hours or days after intravitreal injection that was not detectable 9 days after injection. Therefore, extensive functional retinal recovery may have occurred. Finally, this is an experimental study and the limitations of the rabbit model include differences in retinal vascularization and eye volume when compared with the human eye. For these reasons, the results may not fully represent the effects in human eyes.
In conclusion, this pharmacokinetic study suggests that the vitreous half-life of acyclovir is very short, and the clinical and electrophysiological findings suggest that intravitreal delivery of acyclovir 1 mg in 0.1 mL is safe and well tolerated by rabbit retina. Before intravitreal acyclovir can be used as an adjuvant therapy during early ARN to compensate for the delay in systemic acyclovir reaching therapeutic levels in the retina, additional studies are necessary to determine the precise vitreous half-life of acyclovir, and dose-escalating studies are necessary to determine safe doses for intravitreal injection.
Financial support: Francisco Max Damico (CNPq Postdoctoral Fellowship -150614/2009-8). Mariana Ramos Scolari (FAPESP Undergraduate Research Fellowship -2010/08331-8). Dora Fix Ventura (FAPESP Research Grants -2011/06924-4, 2008/58731-2, and CNPq Fellowship 1A).
AUTHOR CONTRIBUTIONS
Damico FM contributed to the study design, collection, analysis, and interpretation of the data, manuscript writing, acquisition of financial support and general supervision of the research group. Scolari MR, Ioshimoto GL, Takahashi BS, Fialho SL, Bonci DM, and Gasparin F were responsible for data collection. Cunha Jr. AS contributed to the study design, collection, analysis and interpretation of the data, and drafting of the manuscript. Ventura DF contributed to the conception and design of the study, analysis and interpretation of the data, writing of the manuscript, acquisition of financial support and general supervision of the research group.
Received for publication on February 23, 2012
First review completed on March 18, 2012
Accepted for publication on April 1, 2012
E-mail: fmdamico@usp.br
Tel.: 55 11 3212-0200
References
- 1. Blumenkranz MS, Culbertson WW, Clarkson JG, Dix R. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology. 1986;93(3):296-300.
- 2. Fisher JP, Lewis ML, Blumenkranz M, Culbertson WW, Flynn HW, Jr., Clarkson JG, et al. The acute retinal necrosis syndrome. Part 1: Clinical manifestations. Ophthalmology. 1982;89(12):1309-16.
- 3. Crapotta JA, Freeman WR, Feldman RM, Lowder CY, Ambler JS, Parker CE, et al. Visual outcome in acute retinal necrosis. Retina. 1993;13(3):208-13.
- 4. Schaeffer HJ, Beauchamp L, de Miranda P, Elion GB, Bauer DJ, Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978;272(5654):583-5.
- 5. Rosenberry KR, Bryan CK, Sohn CA. Acyclovir: evaluation of a new antiviral agent. Clin Pharm. 1982;1(5):399-406.
- 6. Sasaki H, Yamamura K, Mukai T, Nishida K, Nakamura J, Nakashima M, et al. Enhancement of ocular drug penetration. Crit Rev Ther Drug Carrier Syst. 1999;16(1):85-146.
- 7. Sims JL, Yeoh J, Stawell RJ. Acute retinal necrosis: a case series with clinical features and treatment outcomes. Clin Experiment Ophthalmol. 2009;37(5):473-7.
- 8. Bahrami G, Mohammadi B. An isocratic high performance liquid chromatographic method for quantification of mycophenolic acid and its glucuronide metabolite in human serum using liquid-liquid extraction: application to human pharmacokinetic studies. Clin Chim Acta. 2006;370(1-2):185-90.
- 9. Khoschsorur G, Erwa W. Liquid chromatographic method for simultaneous determination of mycophenolic acid and its phenol-and acylglucuronide metabolites in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;799(2):355-60.
- 10. Watson DG, Araya FG, Galloway PJ, Beattie TJ. Development of a high pressure liquid chromatography method for the determination of mycophenolic acid and its glucuronide metabolite in small volumes of plasma from paediatric patients. J Pharm Biomed Anal. 2004;35(1):87-92.
- 11. Pastore A, Lo Russo A, Piemonte F, Mannucci L, Federici G. Rapid determination of mycophenolic acid in plasma by reversed-phase highperformance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;776(2):251-4.
- 12. Kim H, Csaky KG, Chan CC, Bungay PM, Lutz RJ, Dedrick RL, et al. The pharmacokinetics of rituximab following an intravitreal injection. Exp Eye Res. 2006;82(5):760-6.
- 13. Kim H, Csaky KG, Gravlin L, Yuan P, Lutz RJ, Bungay PM, et al. Safety and pharmacokinetics of a preservative-free triamcinolone acetonide formulation for intravitreal administration. Retina. 2006;26(5):523-30.
- 14. Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M, et al. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009;118(1):69-77.
- 15. Harazny J, Scholz M, Buder T, Lausen B, Kremers J. Electrophysiological deficits in the retina of the DBA/2J mouse. Doc Ophthalmol. 2009;119(3):181-97.
- 16. Perlman I. Testing retinal toxicity of drugs in animal models using electrophysiological and morphological techniques. Doc Ophthalmol. 2009;118(1):3-28.
- 17. Asi H, Perlman I. Relationships between the electroretinogram a-wave, b-wave and oscillatory potentials and their application to clinical diagnosis. Doc Ophthalmol. 1992;79(2):125-39.
- 18. Frishman L. Origins of the electroretinogram. In: Heckenlively J, Arden G, editors. Principles and practice of clinical electrophsyiology of vision. 2 ed. Cambridge, USA: The MIT Press; 2006. p.139-84.
- 19. Hebert M, Lachapelle P, Dumont M. Reproducibility of electroretinograms recorded with DTL electrodes. Doc Ophthalmol. 1995;91(4):333-42.
- 20. Marmor MF, Holder GE, Seeliger MW, Yamamoto S, , International Society for Clinical Electrophysiology of V. Standard for clinical electroretinography (2004 update). Doc Ophthalmol. 2004;108(2):107-14.
- 21. Xu W, Wang H, Wang F, Jiang Y, Zhang X, Wang W, et al. Testing toxicity of multiple intravitreal injections of bevacizumab in rabbit eyes. Can J Ophthalmol. 2010;45(4):386-92.
- 22. Inan UU, Avci B, Kusbeci T, Kaderli B, Avci R, Temel SG. Preclinical safety evaluation of intravitreal injection of full-length humanized vascular endothelial growth factor antibody in rabbit eyes. Invest Ophthalmol Vis Sci. 2007;48(4):1773-81.
- 23. Gao H, Pennesi ME, Qiao X, Iyer MN, Wu SM, Holz ER, et al. Intravitreal moxifloxacin: retinal safety study with electroretinography and histopathology in animal models. Invest Ophthalmol Vis Sci. 2006;47(4):1606-11.
- 24. Schulman J, Peyman GA, Horton MB, Liu J, Barber JC, Fiscella R, et al. Intraocular penetration of new antiviral agent, hydroxyacyclovir (BWB759U). Jpn J Ophthalmol. 1986;30(1):116-24.
- 25. Pulido J, Peyman GA, Lesar T, Vernot J. Intravitreal toxicity of hydroxyacyclovir (BW-B759U), a new antiviral agent. Arch Ophthalmol. 1985;103(6):840-1.
- 26. Pulido JS, Palacio M, Peyman GA, Fiscella R, Greenberg D, Stelmack T. Toxicity of intravitreal antiviral drugs. Ophthalmic Surg. 1984;15(8):666-9.
- 27. Brigden D, Whiteman P. The mechanism of action, pharmacokinetics and toxicity of acyclovira review. J Infect. 1983;6(1 Suppl):3-9.
- 28. Poirier JM, Radembino N, Jaillon P. Determination of acyclovir in plasma by solid-phase extraction and column liquid chromatography. Ther Drug Monit. 1999;21(1):129-33.
- 29. Good SS, de Miranda P. Metabolic disposition of acyclovir in the guinea pig, rabbit, and monkey. Am J Med. 1982;73(1A):91-5.
- 30. Pellegatti M, Pagliarusco S, Solazzo L, Colato D. Plasma protein binding and blood-free concentrations: which studies are needed to develop a drug? Expert Opin Drug Metab Toxicol. 2011;7(8):1009-20.
Publication Dates
-
Publication in this collection
30 Aug 2012 -
Date of issue
Aug 2012
History
-
Received
23 Feb 2012 -
Accepted
01 Apr 2012 -
Reviewed
18 Mar 2012

 Vitreous pharmacokinetics and electroretinographic findings after intravitreal injection of acyclovir in rabbits
Vitreous pharmacokinetics and electroretinographic findings after intravitreal injection of acyclovir in rabbits