Abstract
OBJECTIVES: Idiopathic central precocious puberty and its postponement with a (gonadotropin-releasing hormone) GnRH agonist are complex conditions, the final effects of which on bone mass are difficult to define. We evaluated bone mass, body composition, and bone remodeling in two groups of girls with idiopathic central precocious puberty, namely one group that was assessed at diagnosis and a second group that was assessed three years after GnRH agonist treatment. METHODS: The precocious puberty diagnosis and precocious puberty treatment groups consisted of 12 girls matched for age and weight to corresponding control groups of 12 (CD) and 14 (CT) girls, respectively. Bone mineral density and body composition were assessed by dual X-ray absorptiometry. Lumbar spine bone mineral density was estimated after correction for bone age and the mathematical calculation of volumetric bone mineral density. CONEP: CAAE-0311.0.004.000-06. RESULTS: Lumbar spine bone mineral density was slightly increased in individuals diagnosed with precocious puberty compared with controls; however, after correction for bone age, this tendency disappeared (CD = -0.74 + 0.9 vs. precocious puberty diagnosis = -1.73 + 1.2). The bone mineral density values of girls in the precocious puberty treatment group did not differ from those observed in the CT group. CONCLUSION: There is an increase in bone mineral density in girls diagnosed with idiopathic central precocious puberty. Our data indicate that the increase in bone mineral density in girls with idiopathic central precocious puberty is insufficient to compensate for the marked advancement in bone age observed at diagnosis. GnRH agonist treatment seems to have no detrimental effect on bone mineral density.
Precocious puberty; Osteoporosis in children; Bone density; Gonadotropin-Releasing Hormone
CLINICAL SCIENCE
Bone mineral density and body composition in girls with idiopathic central precocious puberty before and after treatment with a gonadotropin-releasing hormone agonist
Sandra B. AlessandriI; Francisco de A. PereiraI; Rosângela A. VillelaI; Sonir R. R. AntoniniII; Paula C. L. EliasI; Carlos E. Martinelli Jr.II; Margaret de CastroI; Ayrton C. MoreiraI; Francisco J. A. de PaulaI
IUniversidade de São Paulo, School of Medicine of Ribeirão Preto, Department of Internal Medicine, Ribeirão Preto/SP, Brazil
IIUniversidade de São Paulo, School of Medicine of Ribeirão Preto, Department of Pediatrics, Ribeirão Preto/SP, Brazil
ABSTRACT
OBJECTIVES: Idiopathic central precocious puberty and its postponement with a (gonadotropin-releasing hormone) GnRH agonist are complex conditions, the final effects of which on bone mass are difficult to define. We evaluated bone mass, body composition, and bone remodeling in two groups of girls with idiopathic central precocious puberty, namely one group that was assessed at diagnosis and a second group that was assessed three years after GnRH agonist treatment.
METHODS: The precocious puberty diagnosis and precocious puberty treatment groups consisted of 12 girls matched for age and weight to corresponding control groups of 12 (CD) and 14 (CT) girls, respectively. Bone mineral density and body composition were assessed by dual X-ray absorptiometry. Lumbar spine bone mineral density was estimated after correction for bone age and the mathematical calculation of volumetric bone mineral density. CONEP: CAAE-0311.0.004.000-06.
RESULTS: Lumbar spine bone mineral density was slightly increased in individuals diagnosed with precocious puberty compared with controls; however, after correction for bone age, this tendency disappeared (CD = -0.74 + 0.9 vs. precocious puberty diagnosis = -1.73 + 1.2). The bone mineral density values of girls in the precocious puberty treatment group did not differ from those observed in the CT group.
CONCLUSION: There is an increase in bone mineral density in girls diagnosed with idiopathic central precocious puberty. Our data indicate that the increase in bone mineral density in girls with idiopathic central precocious puberty is insufficient to compensate for the marked advancement in bone age observed at diagnosis. GnRH agonist treatment seems to have no detrimental effect on bone mineral density.
Keywords: Precocious puberty; Osteoporosis in children; Bone density; Gonadotropin-Releasing Hormone.
INTRODUCTION
Although the mechanistic process of bone mass gain remains to be clarified, it is recognized that genetic factors determine 60 to 80% of peak bone mass and that sex steroids and growth factors play a pivotal role in the ascending and descending shape of the bone mineral density (BMD) curve (1,2).
BMD increases significantly during puberty; it is accepted that approximately 40% of peak bone mass is acquired between Tanner stages II and V (1,3) and that the rate of acquisition is particularly high between stage III and stage IV (4,5). Thereafter, bone acquisition decelerates, and the increase continues at a slow rate until bone consolidation has been completed (6). Gonadal steroids can affect bone mass acquisition either directly or indirectly through effects on molecules such as growth hormone, insulin-like growth factor-1, and 1,25(OH)2 D (6-9). Under normal circumstances, pubertal gonadotropin production in females leads to the increased conversion of C19 steroids (androstenedione and testosterone) to estrogens, which in turn results in growth acceleration, skeletal maturation and epiphyseal fusion (10). BMD at the radius and lumbar spine levels has been found to be significantly higher in girls with precocious puberty compared with prepubertal girls (11,12). Moreover, delayed puberty is associated with reduced bone mass (13-15).
The limited accrual of peak bone mass is so important for the determination of future bone strength that osteoporosis has been considered a pediatric disorder with repercussions (e.g. occurence of fracture) in the elderly population (16,17). Idiopathic central precocious puberty (iCPP) is a frequent disorder of gonadal axis development in girls. iCPP and its treatment with a long-acting agonist of gonadotrophinreleasing hormone (GnRHa) constitute a complex condition that includes factors related to both early exposure to gonadal steroids and a potential delay in the occurrence of puberty. The consequences of iCPP treatment with regard to bone mass accrual and body composition in girls remain to be defined. Previous studies have shown conflicting results; while some have detected negative effects of GnRHa on BMD (11,18), others have shown no detrimental effects (19,20). However, it is necessary to consider that the cited studies evaluated bone mass over a short period of time or included a heterogeneous group of patients, i.e., boys, girls and patients with organic CPP and with early puberty (19), in addition to individuals with iCPP.
The aim of the present study was to evaluate the early impact of iCPP on the BMD of treatment-naive girls and the long-term influence of depot GnRHa on the BMD and body composition in patients who had completed treatment at least three years previously.
MATERIALS AND METHODS
Subjects
The study included 50 girls divided into 4 groups: a) 12 girls recently diagnosed with iCPP (PPD), b) 12 pre-pubertal control girls matched for age and weight to PPD girls (CD), c) 12 girls previously diagnosed with iCPP who had completed treatment with GnRHa at least 3 years before participating in the study (PPT), and d) 14 adolescent girls who were matched for age and weight to PPT girls (second control group; CT). The mean ages in the CD, PPD, CT, and PPT groups were 8.6 + 1.2, 8.3 + 0.6, 18.6 + 2.8, and 17.6 + 2.6 years, respectively. The study was approved by the Ethics Committee of the University Hospital, School of Medicine of Ribeirão Preto, University of São Paulo (11462/06). Written informed consent was obtained from all individuals and/or their families after they had been informed about the aims, procedures and risks of the study.
The diagnosis of iCPP was based on the emergence of secondary pubertal signs before the chronological age (CA) of 8 year, acceleration of the growth rate, bone age advance of more than 1 year with regard to the CA, and the analysis of basal and stimulated serum levels of LH. The laboratory test was considered positive when the basal LH level was > 0.6 IU/L and/or > 6.9 IU/L after the GnRH stimulation test (21). All patients with a diagnosis of iCPP underwent a hypothalamic/pituitary MRI evaluation, which yielded normal results. In all groups, children with a personal and/or family history of osteometabolic disease and those taking medications that impact bone mineral metabolism (estrogens, glucocorticoids, diuretics, and anticonvulsants) were excluded. All children admitted to the study had normal kidney and hepatic function. Children with pathological CPP and early pubertal children were excluded. The selected patients were followed at the outpatient clinic of Pediatric Endocrinology Division, University Hospital, School of Medicine of RibeirãoPreto, USP.
Methods
A peripheral blood sample was collected after an overnight fast and immediately processed to obtain serum; aliquots were stored at -70 °C. A second voided urine sample was collected under standardized conditions from 08:0009:00 am. One aliquot was immediately used for creatinine determination, and a second sample was stored at -70 °C.
Serum levels of total calcium, inorganic phosphorus and alkaline phosphatase were determined using an automatic biochemical analyzer (Dimension RXL, Dade-Behring, Deerfield, IL, USA). Osteocalcin was measured using an immunoradiometric (IRMA) method (hOST-IRMA, Biosource Europe, Nivelles, Belgium). Urinary deoxypyridinoline was measured using an immunoenzymatic method (ELISA) (Metra Biosystems, Mountain View, CA, USA). Serum 25-hydroxyvitamin D (25-OHD) and parathyroid hormone (PTH) were measured by radioimmunoassay (Diasorin, Saluggia, VC, Italy) and chemoluminescence (Diagnostic Products Corporation, Los Angeles, CA, USA), respectively. The degrees of intra- and interassay variation were 3.6 and 7.5% for osteocalcin, 4.1 and 6.8% for deoxypyridinoline, 5.4 and 8.6% for 25-OHD, and 3.2 and 5.6% for PTH, respectively.
Scans of lumbar spine (L1-L4), total hip and femoral neck BMD, as well as of total body lean and fat mass, were obtained by double-energy X-ray absorptiometry (Hologic 4500W, USA). The exams were performed by the same operator according to rigid positioning criteria standardized for each target site. The BMD results are reported as areal BMD (g/cm2) and Z-scores. The Z-scores were re-evaluated after adjustment for bone age (BABMD). In addition, volumetric density of the lumbar spine (aBMD) was also estimated mathematically in L2-L4, as proposed by Carter et al. (22), using the following equation: aBMD = α/β, where a is the BMD (in g/cm2) of the lumbar spine (L2-L4) and β is the square root of the bone area (in cm2) of the lumbar spine (L2-L4).
The precision errors of BMD measurements were 1.2% for the lumbar spine (L1-L4), 1.9% for the femoral neck, and 2.9% for the total femur. Low BMD was defined using the criteria of the International Society of Clinical Densitometry, i.e., a Z-score of -2.0 or less adjusted for age and gender (23). Bone age and BABMD were determined as described previously (24).
The results are reported as the means + SD. Statistical analysis was performed by considering two groups each time (e.g., the iCPP and corresponding age-matched control groups). The results were analyzed by the nonparametric Mann-Whitney test, and correlations between parameters were analyzed using the Spearman test. Statistical analysis was performed using GraphPad Prism 3.0 software (1999; GraphPad Prism, San Diego, CA, USA). The level of significance was set at 5% in all analyses.
RESULTS
Children at diagnosis
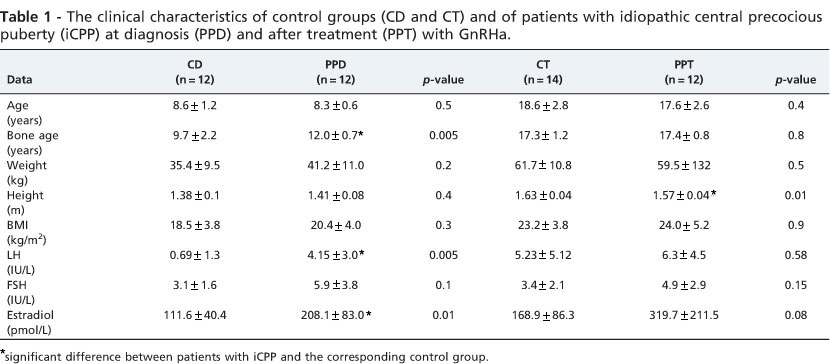
Table 1 shows that there was no significant difference in age, weight, height, or BMI between the CD and PPD groups. However, bone age (BA) was significantly increased in patients diagnosed with iCPP (CD = 9.7+ 2.2 vs. PPD = 12.0 + 0.7 years; p<0.0005). Serum levels of LH and estradiol, but not FSH, were also significantly increased in the PPD group.
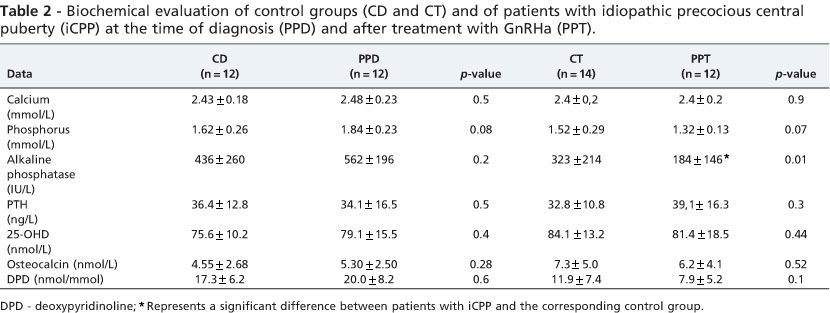
There were no significant differences in serum calcium, phosphorus or alkaline phosphatase, PTH or 25(OH)D levels between CD and PPD patients (Table 2). The biochemical markers of bone formation (osteocalcin) and of bone resorption (deoxypyridinoline) were similar in PPD and CD patients (Table 2).
There was no significant difference in total body BMD between the CD and PPD groups (Table 3). The BMD of the lumbar spine in PPD patients was not significantly higher than that in the corresponding control girls (CD = 0.613 + 0.08 vs. PPD = 0.633 + 0.09 g/cm2). However, when BMD at L1-L4 was expressed as the Z-score corrected for BA, BABMD tended to decrease in children with PPD (CD = -0.74 + 0.9 vs. PPD = -1.73 + 1.2 SD; p<0.06). The estimated volumetric density was similar between the groups (CD = 0.113 + 0.01 vs. PPD = 0.114 + 0.01 g/cm3). Total hip BMD was significantly higher in PPD patients (0.693 + 0.05) compared with the control group (0.625 + 0.05) (p<0.007).
iCPP Girls after GnRHa treatment
Table 1 shows that there were no significant differences in age, weight or BMI between the group of patients previously treated with GnRHa and the control group, but height was significantly higher in CT (1.63+ 0.04 m) than in PPT (1.57 + 0.04 m) patients (p<0.01).
The serum levels of pituitary/gonadal axis hormones (LH, FSH and estradiol) did not differ significantly between groups. Serum alkaline phosphatase levels were significantly lower in the PPT group (184 + 146 IU/L) than in the CT group (323 + 214 IU/L) (p<0.01), but there was no significant difference between groups in the serum levels of calcium, phosphorus, PTH or 25(OH)D (Table 2). Serum osteocalcin levels and urinary DPD levels were slightly lower in PPT compared with CT patients.
There was no difference in lumbar spine BMD between the PPT and CT groups, and the same pattern was observed in both groups after the estimation of volumetric BMD. Additionally, femoral neck and total hip BMD values were also similar in these groups (Table 4).
Adolescents with iCPP previously treated with GnRHa exhibited body composition parameter values similar to those of the control group, i.e., %FM was not significantly affected by the previous use of GnRHa (CT = 34.9 + 5.1% vs. PPT = 35.0 + 6.7%) (Table 3).
DISCUSSION
Physiological and pathological fluctuations in circulating estrogen levels are associated with a positive or negative net imbalance in bone remodeling (25-27). The onset of puberty represents a unique opportunity for the extra-uterine acceleration of bone mass gain, and it is well known that delayed puberty is associated with impaired bone mass accrual. Conversely, the final effect of the postponement of the pubertal surge provoked by GnRHa in bone mass development in girls diagnosed with iCPP remains to be clarified. The present study shows that, at diagnosis, iCPP patients tend toward a higher BMD than their correspondent control individuals. However, advancement in bone age is proportionally greater than the increase in bone mass in these patients; accordingly, BABMD in the lumbar spine was almost significantly lower in recently diagnosed iCPP patients. Additionally, the present data show that the BMD in patients who had concluded treatment at least three years prior to enrollment in the study was analogous to that of the corresponding controls.
Previous studies have shown that BMD was significantly increased in patients with iCPP and that GnRHa treatment for 2 years impaired bone mass acquisition in these patients (18,28). The authors also observed that bone loss was preventable by calcium supplementation (18). In contrast, in a more recent study, it was observed that patients with iCPP exhibited an increase in BMD one year after treatment with GnRHa (29). In addition, a previous study observed that BMD corrected for bone age was decreased in iCPP patients and that this BMD difference decreased after GnRHa treatment. Similar to previous reports, we observed that the tendency for iCCP patients to exhibit high absolute BMD disappeared when the result was adjusted for BA. However, our data are not directly comparable to those reported in the studies described above. Whereas the authors of previous studies evaluated the immediate effect of GnRHa on BMD, we determined BMD 3 years after the conclusion of treatment. Therefore, the present investigation is the first to show that long-term GnRHa treatment allows iCPP girls to improve their acquisition of bone mass. The insignificant difference in L1-L4 BMD between iCPP adolescents and controls vanished after the estimation of volumetric BMD.
Total hip BMD was significantly higher in iCPP children than in controls. Recently, it was hypothesized that bone loss at cortical sites is more closely related to estrogen levels than bone loss in trabecular bone (30). In support of this proposal, observations based on quantitative computed tomography showed that the decrease in trabecular bone mass precedes a decrease in serum estrogen levels, while cortical bone loss coincides with the occurrence of hypoestrogenemia (30,31). Our results do not allow us to conclude that precocious puberty favors cortical bone gain to the detriment of trabecular bone. Compared with the control group, girls recently diagnosed with iCPP showed a slight increase in BMD at L1-L4, which predominantly reflected an increase in trabecular bone, and significantly increased BMD in the total hip, which is composed of a mixture of trabecular and cortical bone. In parallel, BMD at the distal 1/3 of the forearm, which consists mainly of cortical bone, was similar in iCPP girls and controls. GnRHa therapy did not interfere with bone mass gain in bone composed of distinct mixtures of trabecular and cortical bone. BMD values in the lumbar spine, distal 1/3 of the forearm and the proximal femur of iCPP adolescents submitted to this treatment were analogous to control values.
Evidence connecting bone to adipose tissue and both to the endocrine control of intermediary metabolism has emerged in recent years. Osteocalcin from osteoblasts and leptin and adiponectin from adipocytes are some of the molecules involved in these new networks (32-34). Curiously, pubertal onset seems to be closely related to adipose tissue volume, meaning that leptin may be a link between potential alterations in skeletal and intermediary metabolism associated with pubertal onset (35). Therefore, there is great interest in the study of body composition in iCPP, particularly in the fat compartment due to its potential effects on insulin sensitivity. Our results show that neither children with the diagnosis of iCPP nor adolescents who have received long-term treatment with GnRHa display alterations in body mass composition. Moreover, %FM in the group of adolescents previously treated with GnRHa was equivalent to that of the control group. In a recent clinical investigation, it was suggested that carboxylated osteocalcin and undercarboxylated osteocalcin are both associated with insulin sensitivity. Our results show that serum osteocalcin levels in patients with iCPP before and after treatment are similar to those of their corresponding control groups.
This study has some limitations. The number of patients studied was insufficient to definitively exclude the impact of GnRHa on bone in individuals with iCPP. Additionally, our study has a cross-sectional design and did not allow for the estimation of an increase in bone mass during GnRHa therapy. However, these are important preliminary data for a more comprehensive study examining the impact of iCPP and its treatment on bone mass development.
In summary, our results show that BMD is not particularly affected by iCPP in girls and that the treatment of iCPP with GnRHa does not seem to have a detrimental effect on the acquisition of bone mass. We also observed that iCPP has no remarkable effect on body composition. The limitation of growth rate continues to be the major issue to be improved in the treatment of girls who have precociously entered puberty.
ACKNOWLEDGMENTS
This study was supported by the Fundação de Apoio a Pesquisa do Estado de São Paulo (61398-8, FAPESP). SBA received financial support from FAPESP, and we thank Sebastião Brandão Filho for technical assistance with the laboratory assays.
AUTHOR CONTRIBUTIONS
Alessandri SB, Pereira FA and Vilella RA participated in sample collections and laboratory measurements. Anotinini SRR, Elias PCL and Martinelli CE were responsible for patient selection and the laboratory diagnostics for iCPP. Moreira AC, Castro M and Paula FJA were responsible for the study design and data analysis. Paula FJA wrote the manuscript, which was revised by all authors.
Received for publication on January 24, 2012
First review completed on February 27, 2012
Accepted for publication on February 27, 2012
E-mail: fjpaula@fmrp.usp.br
Tel.: 55 16 3602-2563
References
- 1. Clarke BL, Khosla S. Female reproductive system and bone. Arch Biochem Biophys. 2010;503(1):118-28, http://dx.doi.org/10.1016/j.abb.2010.07.006
- 2. Norris SA, Nelson DA. Ethnic differences in bone acquisition. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7th ed. Washington: Wiley, JW. 2008.p.80-2.
- 3. Saggese G, Bertelloni S, Baroncelli GI. Sex steroids and the acquisition of bone mass. Horm Res. 1997;48(Suppl 5):65-71, http://dx.doi.org/10.1159/000191331
- 4. Grimston SK, Morrison K, Harder JA, Hanley DA. Bone mineral density during puberty in western Canadian children. Bone Miner. 1992;19(1):85- 96, http://dx.doi.org/10.1016/0169-6009(92)90846-6
- 5. Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93(2):799-808, http://dx.doi.org/10.1172/JCI117034
- 6. Holmes SJ, Shalet SM. Role of growth hormone and sex steroids in achieving and maintaining normal bone mass. Horm Res 1996;45(1-2):86-93.
- 7. Krabbe S, Transbol I, Christiansen C. Bone mineral homeostasis, bone growth, and mineralisation during years of pubertal growth: a unifying concept. Arch Dis Child. 1982;57(5):359-63, http://dx.doi.org/10.1136/adc.57.5.359
- 8. Spelsberg TC, Subramaniam M, Riggs BL, Khosla S. The actions and interactions ofsex steroids and growth factors/cytokines onthe skeleton. Mol Endocrinol. 1999;13(6):819-28, http://dx.doi.org/10.1210/me.13.6.819
- 9. Vanderschueren D. Androgens and their role in skeletal homeostasis. Horm Res. 1996;46(2):95-8, http://dx.doi.org/10.1159/000185003
- 10. MacGillivray MH, Morishima A, Conte F, Grumbach M, Smith EP. Pediatric endocrinology update: an overview. The essential roles of estrogens in pubertal growth, epiphyseal fusion and bone turnover: lessons from mutations in the genes for aromatase and the estrogen receptor. Horm Res. 1998;49(Suppl 1):2-8, http://dx.doi.org/10.1159/000053061
- 11. Saggese G, Bertelloni S, Baroncelli GI, Battini R, Franchi G. Reduction of bone density: an effect of gonadotropin releasing hormone analogue treatment in central precocious puberty. Eur J Pediatr. 1993;152(9):717-20, http://dx.doi.org/10.1007/BF01953983
- 12. Takahashi Y, Minamitani K, Kobayashi Y, Minagawa M, Yasuda T, Niimi H. Spinal and femoral bone mass accumulation during normal adolescence: comparison with female patients with sexual precocity and with hypogonadism. J Clin Endocrinol Metab. 1996;81(3):1248-53, http://dx.doi.org/10.1210/jc.81.3.1248
- 13. Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Influence of age at menarche on forearm bone microstructure in healthy young women. J Clin Endocrinol Metab. 2008;93(7):2594-601, http://dx.doi.org/10.1210/jc.2007-2644
- 14. Finkelstein JS, Klibanski A, Neer RM. A longitudinal evaluation of bone mineral density in adult men with histories of delayed puberty. J Clin Endocrinol Metab. 1996;81(3):1152-5, http://dx.doi.org/10.1210/jc.81.3.1152
- 15. Finkelstein JS, Neer RM, Biller BM, Crawford JD, Klibanski A. Osteopenia in men with a history of delayed puberty. N Engl J Med. 1992;326(9):600-4, http://dx.doi.org/10.1056/NEJM199202273260904
- 16. Burrows M, Baxter-Jones A, Mirwald R, Macdonald H, McKay H. Bone mineral accrual across growth in a mixed-ethnic group of children: are Asian children disadvantaged from an early age? Calcif Tissue Int. 2009;84(5):366-78, http://dx.doi.org/10.1007/s00223-009-9236-8
- 17. Golden NH. Osteoporosis prevention: a pediatric challenge. Arch Pediatr Adolesc Med. 2000;154(6):542-3.
- 18. Antoniazzi F, Bertoldo F, Lauriola S, Sirpresi S, Gasperi E, Zamboni G, et al. Prevention of bone demineralization by calcium supplementation in precocious puberty during gonadotropin-releasing hormone agonist treatment. J Clin Endocrinol Metab. 1999;84(6):1992-6, http://dx.doi.org/10.1210/jc.84.6.1992
- 19. Heger S, Partsch CJ, Sippell WG. Long-term outcome after depot gonadotropin-releasing hormone agonist treatment of central precocious puberty: final height, body proportions, body composition, bone mineral density, and reproductive function. J Clin Endocrinol Metab. 1999;84(12):4583-90, http://dx.doi.org/10.1210/jc.84.12.4583
- 20. van der Sluis IM, Boot AM, Krenning EP, Drop SL, de Muinck Keizer-Schrama SM. Longitudinal follow-up of bone density and body composition in children with precocious or early puberty before, during and after cessation of GnRH agonist therapy. J Clin Endocrinol Metab. 2002;87(2):506-12, http://dx.doi.org/10.1210/jc.87.2.506
- 21. Brito VN, Batista MC,Borges MF, Latronico AC, KohekMB,Thirone AC, et al. Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J Clin Endocrinol Metab. 1999;84(10):3539-44, http://dx.doi.org/10.1210/jc.84.10.3539
- 22. Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7(2):137-45.
- 23. Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008;43(6):1115-21, http://dx.doi.org/10.1016/j.bone.2008.08.106
- 24. de Albuquerque Taveira AT, Fernandes MI, Galvao LC, Sawamura R, de Mello Vieira E, de Paula FJ. Impairment of bone mass development in children with chronic cholestatic liver disease. Clin Endocrinol (Oxf). 2007;66(4):518-23.
- 25. Albright F, Bloomberg E, Smith P. Postmenopausal osteoporosis. Transactions of the Association of American Physicians. 1940;55:298-305.
- 26. Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80(12):3689-98, http://dx.doi.org/10.1210/jc.80.12.3689
- 27. Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene ina man. N Engl J Med. 1994;331(2):1056-61, http://dx.doi.org/10.1056/NEJM199410203311604
- 28. Antoniazzi F, Bertoldo F, Zamboni G, Valentini R, Sirpresi S, Cavallo L, et al. Bone mineral metabolism in girls with precocious puberty during gonadotrophin-releasing hormone agonist treatment. Eur J Endocrinol. 1995;133(4):412-7, http://dx.doi.org/10.1530/eje.0.1330412
- 29. Ko JH, Lee HS, Lim JS, Kim SM, Hwang JS. Changes in bone mineral density and body composition in children with central precocious puberty and early puberty before and after one year of treatment with GnRH agonist. Horm Res Paediatr 2011;75(3):174-9.
- 30. Khosla S, Melton LJ3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res 2011;26(3):441-51.
- 31. Riggs BL, Melton Iii LJ, 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19(12):1945-54, http://dx.doi.org/10.1359/jbmr.040916
- 32. Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309-19, http://dx.doi.org/10.1016/j.cell.2010.06.002
- 33. Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11(1):11-22, http://dx.doi.org/10.1016/j.cmet.2009.11.007
- 34. Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105(13):5266-70, http://dx.doi.org/10.1073/pnas.0711119105
- 35. Mantzoros CS, Moschos SJ. Leptin: in search of role(s) in human physiology and pathophysiology. Clin Endocrinol (Oxf). 1998;49(5):551- 67, http://dx.doi.org/10.1046/j.1365-2265.1998.00571.x
Publication Dates
-
Publication in this collection
29 June 2012 -
Date of issue
2012
History
-
Received
24 Jan 2012 -
Accepted
27 Feb 2012 -
Reviewed
27 Feb 2012

 Bone mineral density and body composition in girls with idiopathic central precocious puberty before and after treatment with a gonadotropin-releasing hormone agonist
Bone mineral density and body composition in girls with idiopathic central precocious puberty before and after treatment with a gonadotropin-releasing hormone agonist