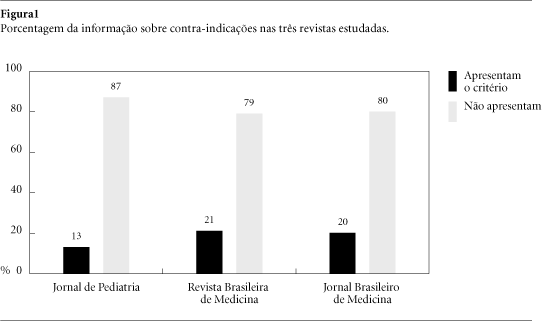
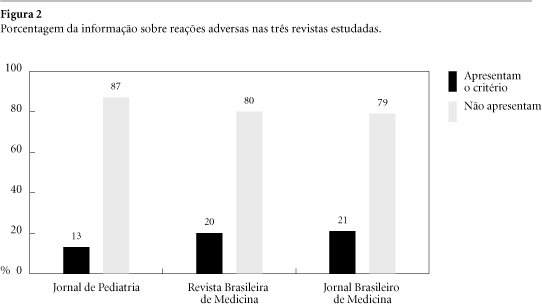
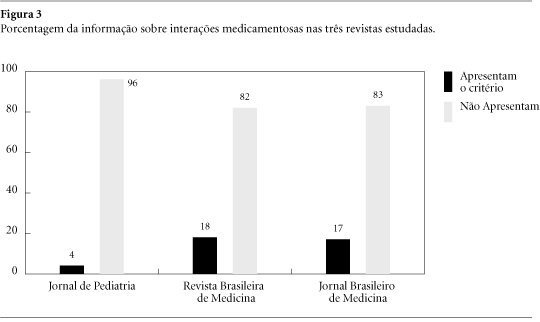
Supply and demand of drugs are influenced by different ways. Manufacturers' promotional strategies are outstanding a particular role being ascribed to advertising (ads) in medical journals. Considering that studies on this issue are not available in Brazil a research was performed aimed at evaluating ads using as parameters WHO criteria for drug information that should be included in any informative material. All advertisements published in Jornal de Pediatria, Revista Brasileira de Medicina and Jornal Brasileiro de Medicina issues from August/2000 up to February/2001 were evaluated verifying the presence of generic name, mechanism of action, pharmacologic effects, indications, contraindications, dosing information, adverse effects, drugs interactions, overdose, types of formulation marketed, manufacturer and/or importer. In the total of 1.774 pages, 539 were occupied by 649 ads. No one of referred criteria was found in all ads and only 20% include contraindications, adverse effects and drugs interactions. The most advertised drugs were antibiotics, antihipertensives and combination products. Instead of subsidize prescription and adequate drug utilization ads are clearly biased obeying to marketing goals since meaningful data are missing.
Drugs advertising; Pharmaceutical industry; Drugs prescription