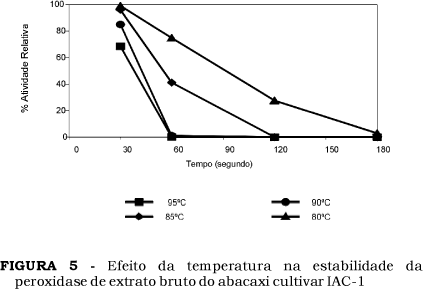
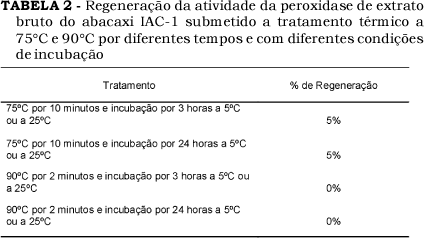
In the present work, the biochemical characteristics of peroxidase enzymes from new pineapples, cultivar IAC Gomo-de-mel and clone IAC-1, were studied. The peroxidases obtained from the juice of this pineapples presented optimum activities from 45 ºC to 50 ºC and 50 ºC to 55 ºC, respectively. These peroxidases showed maximum activity at pH 4.5 and showed stability at pH 4.0 to 9.0, retaining after 24 hours of incubation at 50 ºC more than 80% of initial activity. Partial regeneration of peroxidase activity was observed after treatment at 75 ºC for 10 minutes. The peroxidases from pineapple juices studied were inactivated after treatment at 90 ºC for 2 minutes.
Ananas comosus; pineapple; peroxidase; enzyme inactivation