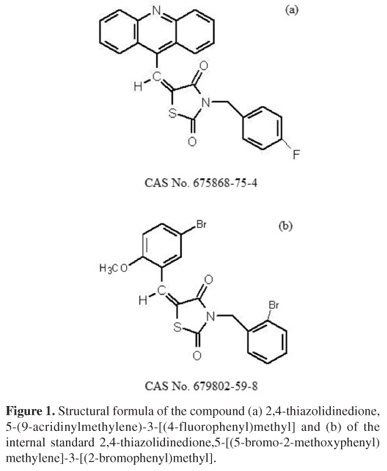
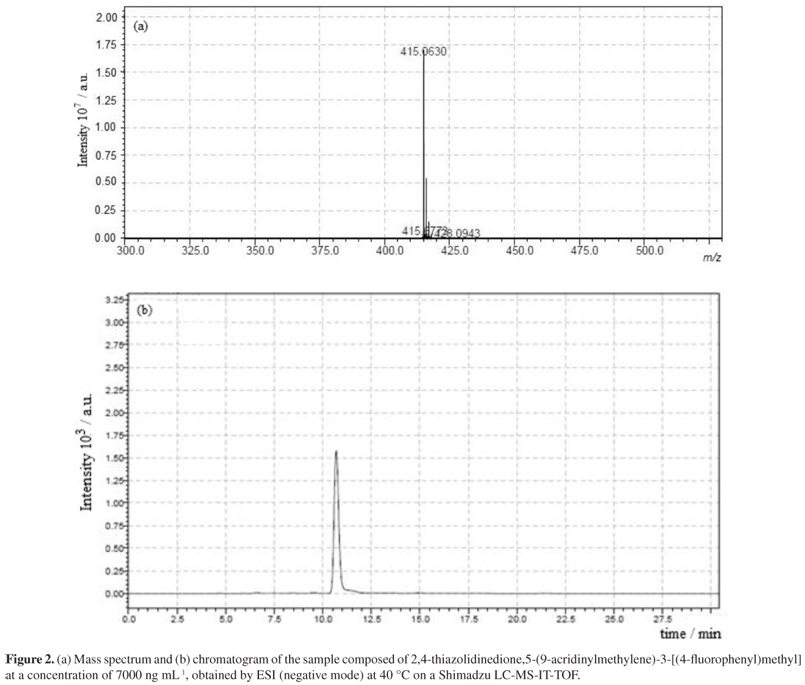
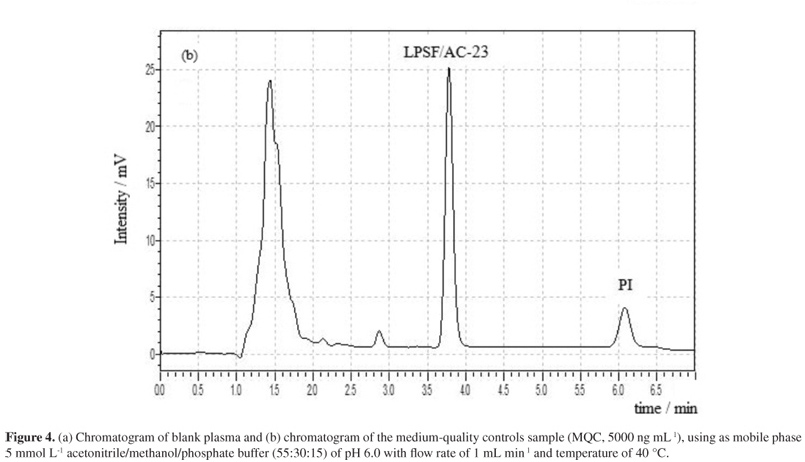
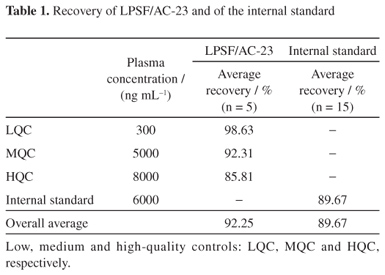
A quick and simple LC-UV (liquid chromatography with ultraviolet detection) method was developed and validated for quantification of 2,4-thiazolidinedione,5-(9-acridinylmethylene)-3-[(4-fluorophenyl)methyl] (LPSF/AC-23) in rat plasma using protein precipitation extraction with acetonitrile, and a thiazolidinedione 2,4-thiazolidinedione,5-[(5-bromo-2-methoxyphenyl)methylene]-3-[(2-bromophenyl)methyl] as an internal standard. The separation and quantification of LPSF/AC-23 were performed using a mobile phase consisting of a mixture of acetonitrile/methanol/phosphate buffer (55:30:15) eluted in an isocratic manner through a C18 analytical column followed by UV detection at 249 nm. The calibration curve was linear in the range of 100-10,000 ng mL-1. The intra- and inter-day precisions expressed by relative standard deviation values were recommended by the Brazilian Health Surveillance Agency (Anvisa), and accuracy expressed by relative error ranged from -3.49 to 7.67%. The recovery was 92.25% for the analyte and 89.67% for the internal standard and no endogenous interference was observed. Thus, the proposed method can be applied to quantitative determination of LPSF/AC-23 in plasma of rats, in pharmacokinetic and bioavailability studies.
LPSF/AC-23; LC-UV; rat plasma; validation; antitumor