Abstracts
The structural organization and histo-cytochemical features of dorsal skin of Ancistrus dolichopterus (acari bodo) are the main focus of this work. The epidermis, dermis and subcutis are the principal layers of the skin. The epidermis mainly consists of epithelial and mucous cells. Interspersed between them are lymphocytes, pigment cells, eosinophilic granular cells (EGC), and the taste buds as sensory structures. The high number of EGCs is implicated in general and specific immunological defense from pathogenic bacteria and multicellular parasites. The epithelial cells and mucous cells contain glycoproteins with oxidizable vicinal diols, carboxyl groups and O-sulphate esters and their high secretory activity is correlated with the bottom dwelling habit of this species. A thick stratum laxum contains overlapping osteoderms bearing denticles, and the stratum compactum make the integument thicker to help the fish in negative buoyancy for maneuvering near the bottom and protection. The entire body surface is covered by conical, backwardly directed denticles. These are composed of a dentine cone, surrounding a pulp cavity with the top covered by mineralized cap, and are the true homologues of teeth. These structures provide effective protection from abrasion and enemies. These structural peculiarities and histochemical features indicate additional physiological role of the skin of A. dolichopterus.
Epidermis; Skin structure; Denticle; Siluriform; Amazon fish
A organização estrutural e aspectos histo-citiquímicos da pele dorsal de Ancistrus dolichopterus (acari-bodó) são os principais alvos do presente estudo. A epiderme, a derme e a hipoderme são as principais camadas da pele. A epiderme consiste principalmente de células epiteliais e mucosas. Intercalados entre elas estão os linfócitos, as células pigmentares, as células granulares eosinofílicas (CGE), e as papilas gustativas como estruturas sensoriais. Um grande número de CGEs está relacionado em geral com a defesa imunológica específica de bactérias patogênicas e parasitas multicelulares. As células epiteliais e as células mucosas contem glicoproteínas com grupos diol oxidáveis, grupos carboxilas e ésteres O-sulfatados sendo que sua alta atividade secretória está correlacionada com o comportamento bentônico, de fundo, dessa espécie. Um espesso stratum laxum contém osteodermos sobrepostos, parecidos com dentículos, e o stratum compactum que torna o tegumento mais espesso, contribuindo com a flutuação negativa necessária ao movimento perto do fundo e proteção. Toda a superfície do corpo é coberta por dentículos cônicos retrodirecionados. Esses dentículos são compostos por um cone de dentina, envolvendo uma cavidade pulpar e com o ápice coberto por uma capa mineralizada, verdadeiros homólogos dos dentes. Essas estruturas oferecem efetiva proteção contra abrasão e oponentes. Essas peculiaridades estruturais e aspectos histoquímicos sugerem a existência de uma função fisiológica adicional para a pele de A. dolichopterus.
ILaboratório de Ecofisiologia e Evolução Molecular, Instituto Nacional de Pesquisas da Amazônia (INPA). Av. André Araújo, 2936, 69060-001 Manaus, AM, Brazil. dalval@inpa.gov.br (corresponding ahthor)
IIDepartment of Zoology, K. N. Government Post-Graduate College, Gyanpur, Bhadohi - 221 304, India
ABSTRACT
The structural organization and histo-cytochemical features of dorsal skin of Ancistrus dolichopterus (acari bodo) are the main focus of this work. The epidermis, dermis and subcutis are the principal layers of the skin. The epidermis mainly consists of epithelial and mucous cells. Interspersed between them are lymphocytes, pigment cells, eosinophilic granular cells (EGC), and the taste buds as sensory structures. The high number of EGCs is implicated in general and specific immunological defense from pathogenic bacteria and multicellular parasites. The epithelial cells and mucous cells contain glycoproteins with oxidizable vicinal diols, carboxyl groups and O-sulphate esters and their high secretory activity is correlated with the bottom dwelling habit of this species. A thick stratum laxum contains overlapping osteoderms bearing denticles, and the stratum compactum make the integument thicker to help the fish in negative buoyancy for maneuvering near the bottom and protection. The entire body surface is covered by conical, backwardly directed denticles. These are composed of a dentine cone, surrounding a pulp cavity with the top covered by mineralized cap, and are the true homologues of teeth. These structures provide effective protection from abrasion and enemies. These structural peculiarities and histochemical features indicate additional physiological role of the skin of A. dolichopterus.
Key words: Epidermis, Skin structure, Denticle, Siluriform, Amazon fish.
RESUMO
A organização estrutural e aspectos histo-citiquímicos da pele dorsal de Ancistrus dolichopterus (acari-bodó) são os principais alvos do presente estudo. A epiderme, a derme e a hipoderme são as principais camadas da pele. A epiderme consiste principalmente de células epiteliais e mucosas. Intercalados entre elas estão os linfócitos, as células pigmentares, as células granulares eosinofílicas (CGE), e as papilas gustativas como estruturas sensoriais. Um grande número de CGEs está relacionado em geral com a defesa imunológica específica de bactérias patogênicas e parasitas multicelulares. As células epiteliais e as células mucosas contem glicoproteínas com grupos diol oxidáveis, grupos carboxilas e ésteres O-sulfatados sendo que sua alta atividade secretória está correlacionada com o comportamento bentônico, de fundo, dessa espécie. Um espesso stratum laxum contém osteodermos sobrepostos, parecidos com dentículos, e o stratum compactum que torna o tegumento mais espesso, contribuindo com a flutuação negativa necessária ao movimento perto do fundo e proteção. Toda a superfície do corpo é coberta por dentículos cônicos retrodirecionados. Esses dentículos são compostos por um cone de dentina, envolvendo uma cavidade pulpar e com o ápice coberto por uma capa mineralizada, verdadeiros homólogos dos dentes. Essas estruturas oferecem efetiva proteção contra abrasão e oponentes. Essas peculiaridades estruturais e aspectos histoquímicos sugerem a existência de uma função fisiológica adicional para a pele de A. dolichopterus.
Introduction
Amazonian fishes constitute the most diverse ichthyofauna on the earth; more than 3,000 fish species have been already described in the Amazon, including representatives of almost all freshwater fish groups (de Pinna, 2006). These fishes colonize nearly all types of aquatic habitats in the immense basin (Val & Almeida-Val, 1995). For that they developed a suite of morpho-physiological adaptations that includes the latest frontier between internal and external media, the skin. In fishes, the skin is involved on the protection against outside physical and chemical agents as well as pathogens. The basic structure is similar among different groups, but specific morphological and cytological features can be observed. The comparative anatomy of fish skin, especially the epidermis, has been investigated by light and electron microscopy (for reviews, see Imaki & Chavin, 1984; Whitear, 1986). Bhatti (1938) made an elaborate study of the dermal skeleton when he studied the integument of Siluroidea. Sire & Meunier (1993) described only the superficial ornamentation and structure of the dermal plates in a few armored Siluriformes.
The siluriforms comprise about 27% of the neotropical fish species (Reis et al., 2003). Among them the largest family of neotropical catfishes, the Loricariidae. The "acari bodo" Ancistrus dolichopterus (Kner, 1854), is a member of the largest subfamily, of Loricariidae, the Ancistrinae, which presents 217 species (Reis et al., 2003). Ancistrus dolichopterus is a bristle-nosed or bushy-faced catfish, sluggish, hardy fish that inhabits fresh to brackish waters: it is a bottom dweller, generally clinging to rocks, logs or other objects through suckermouth (Burgess, 1989). As the skin is in constant contact with the environment and is also an important barrier to pathogens, the present investigation reports the structural organization and histo-cytochemical features in relation to the functional significance of the dorsal skin of Ancistrus dolichopterus.
Material and Methods
Live specimens (n = 20) of Ancistrus dolichopterus (reference specimen INPA-25626; length 12.5 ± 1.0 cm; weight 45.7 ± 4.8 g) were collected from Solimões River (Catalão) at Manaus, Brazil. The fish were maintained for four weeks in 500 l aerated outdoor tanks in the laboratory and were fed with pelleted food on alternate days. The fish were acclimated to the natural diurnal light-dark cycle of 14-10 h.
Fish were cold anaesthetized following Mittal & Whitear (1978). Skin pieces, from the dorsum side close to the dorsal fin, were excised, rinsed in physiological saline and fixed in aqueous Bouin's fluid, alcoholic Bouin's fluid and 10% neutral formalin. After fixation the samples were treated with 5% formic acid for 24 h and were then washed with running tap water for 2 h. Tissues were dehydrated in graded series of ethyl alcohol, cleared in cedar wood oil and embedded in paraffin wax.
Paraffin sections were cut at 6 mm and were stained with Ehrlich's haematoxylin-eosin (HE), Verhoeff's haematoxylin (VHE) and Papanicolaou stain (PS) following Bancroft and Stevens (1982) for evaluation of general organization. To assess carbohydrate histochemistry, slides were stained with Schiff without prior oxidation to demonstrate free aldehydes; with periodic acid-Schiff (PAS) with or without prior acetylation to localize glycoproteins (or mucopolysaccharides) with oxidizable vicinal diols and/or glycogen; with deacetylation-PAS to hydrolyze the acetyl esters and to unblock the reactive hydroxyl groups; with ptyalin (saliva) digestion followed by PAS, and Best carmine to distinguish the glycoproteins with oxidizable vicinal diols from glycogen contents; with alcian blue (AB) at pH 2.5 to localize glycoproteins with carboxyl groups and/or with O-sulphate esters; with prior methylation-AB pH 2.5 to block the glycoproteins with carboxyl groups and/or with O-sulfate esters; with prior methylation-saponification-AB pH 2.5 to discriminate between the glycoproteins with carboxyl group or O-sulfate esters; with AB at pH 1.0 to identify the glycoproteins with O-sulfate esters only; with combined AB-PAS to differentiate glycoproteins with oxidizable vicinal diols and/or glycogen, and glycoproteins with carboxyl groups and/or with O-sulphate esters; with methylation-AB pH 2.5-PAS to block the carboxyl groups and/or with O-sulphate esters with simultaneous observation of glycoproteins with oxidizable vicinal diols and with prior methylation-saponification-AB pH2.5-PAS to demonstrate the glycoproteins with oxidizable vicinal diols and/or glycogen and to specify these with carboxyl groups or O-sulfate esters following Bancroft & Stevens (1982) and Pearse (1985).
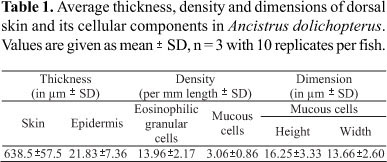
Stage micrometer and eyepiece measuring disc (Carl Zeiss, Jena) were used to count the number of eosinophilic granular cells and mucous cells per mm length of the epidermis, to measure the height and width of mucous cells and to measure the thickness of the epidermis and skin in cross sections.
For each estimation, samples of 10 randomly selected sites from each animal were analyzed. The results are expressed as mean ± SD throughout.
Results
The skin of Ancistrus dolichopterus consists of a multilayered epidermis, dermis, and subcutis (Fig. 1A). The average thickness of the skin is 638.5 ± 157.5 mm. Furthermore, the osteoderms recessed into the skin with their embedded denticles which are distributed over the entire surface of the body (Figs. 1B, 1C).
Epidermis
Measurements of epidermis, four to six cell layers thick, are summarized in the Table 1. The main structural component of the epidermis is epithelial cells, interspersed in between glandular-mucous cells and migrating lymphocytes and eosinophilic granular cells. Pigment cells are also discernible. Club cells and sensory structures such as the ampullary organs (=pit organs), which are the characteristic features of catfish skin, could not be located in the dorsal skin.
Epithelial cells. The basal layer of epithelial cells, arranged in a single layer on a thin non-cellular basement membrane, are usually low columnar in shape with centrally placed rounded nuclei (Fig. 1D), sometimes appearing flattened with flat nuclei. The middle layer epithelial cells, which are arranged less compactly in two to four layers, are, in general, polygonal with centrally placed, rounded nuclei. Epithelial cells, which are below mucous or eosinophilic granular cells, appear flattened with flat nuclei. In the superficial layer, epithelial cells present a flattened aspect with flat nuclei (Fig. 1D). Occasionally, exfoliated epithelial cells isolated or in groups, are also seen on the surface.
Epithelial cells, in general, presented healthy appearing nuclei with distinct chromatin material and nucleoli. They stained dark blue in HE and PS, and blue black in VHE. The cytoplasm stains homogeneously light pink in HE, PS and VHE. Although different histochemical procedures were employed, epithelial cells in the basal layer and lower middle layer remained unstained. Cells in the superficial and outer middle layer stain lightly magenta with PAS, greenish blue with AB (pH 2.5 and 1.0) and purple with AB-PAS (Fig. 1E). These reactions indicate the presence of glycoproteins with oxidizable vicinal diols and glycoproteins with carboxyl groups and/or with O-sulphate esters in low concentrations at these sites. A gradual increase on the intensity of the reactions was detected towards the surface. In A. dolichopterus, a high level of mucogenicity has been demonstrated and this derives from the active involvement of superficial and middle layers of epithelial cells and their voluminous mucous secretion, as indicated by strong mucopolysaccharides reaction (Table 2).
Mucous cells. The density of mucous cells in Ancistrus dolichopterus is remarkably low. Their number, height and width are summarized in Table 1. Sparsely distributed mucous cells are restricted to the outer epidermal layers. These cells are rounded or oval, attain voluminous size, and open on the surface to void their secretions. As the secretory contents accumulate within intracellular vesicles, the remaining cytoplasm, including the flattened nucleus, is pushed basally, towards the periphery, resulting in a strong basophilic unit (Fig. 1E). The flocculent secretory contents are basophilic and stain deep blue with HE and PS. Mucous cells contents stain magenta with PAS with or without prior ptylin treatment, greenish blue with AB (pH 2.5 and 1.0), purple with AB-PAS (Fig. 1E) and methylation-saponification-PAS-AB pH 2.5, and magenta with methylation-PAS-AB pH 2.5, indicating the presence of glycoproteins with oxidizable vicinal diols, carboxyl groups, and O-sulphate esters.
Lymphocytes. Rounded or irregular lymphocytes, usually distributed in the intercellular spaces of the deeper epidermal layers of other fishes (Mittal & Munshi, 1971), were observed sparsely in this fish and stain deep blue in HE, PS and black in VHE.
Pigment cells (melanocytes). Showed well developed, highly branched, and are distributed randomly throughout the deeper layers of the epidermis and in the dermis. These cells are most evident as an almost continuous layer just beneath the basement membrane (Fig. 1D). The pigment cells are filled with dark brown or black coarse granules.
Eosinophilic granular cells. A large number of very well developed eosinophilic granular cells (EGC), spherical or ovoid, are present in the upper, and occasionally in the lower layers of the epidermis. Average density of these cells is summarized in Table 1. These cytoplasmic granules stain bright red in HE, pinkish-red in Verhoeff's elastin stain, orange-red in Papanicolaou's stain and deep red with alizarin red S. The nucleus is eccentrally placed, usually basally, and is flattened with distinct chromatin material and a nucleolus (Fig. 1D). In contrast to several other previous reports these cells stain deep magenta with PAS and AB-PAS (Fig. 1E). However, these cells could not be located in the deeper layers of the dermis and the subcutis.
Taste buds. Very few taste buds could be located on the skin surface. These are pear-shaped structures consisting of spindle shaped neuro-epithelial cells and supporting cells each positioned above a well-developed dermal papilla (Fig. 1F). Long taste hairs arise on the free surface from these neuroepithelial cells.
Dermis
The dermis is about 15-20 times as thick as the epidermis and hence is the largest component of the dorsal skin of Ancistrus. It consists of two distinct layers - the outer stratum laxum and the inner stratum compactum (Fig. 1A).
Stratum laxum. Adjacent to the basement membrane (Fig. 1A) and composed of comparatively loosely arranged connective tissue fibers, mainly collagen, and richly supplied with fine blood capillaries, nerves and pigment cells. The anteriorly-directed elliptical osteoderms are embedded in the stratum laxum and overlap partly the next osteoderm posteriorly. Two horizontal osteoderms are attached with attachment fibers (Fig. 1C). The osteoderms are hollow and have cup shaped projections towards the epidermis for firm anchoring of the denticles (Fig. 1C). An amorphous acellular material may be recognized where the osteoderms are lodged. These areas are stained light pink in HE, greenish blue with AB at pH 1.0 and 2.5, magenta with PAS, and purple with AB-PAS. These histochemical reactions indicate that glycoproteins with oxidizable vicinal diols, carboxyl groups and with O-sulphate esters are present in low concentrations.
Stratum compactum. Comparatively thin (Fig. 1A), this tissue consists of layers of coarse collagen fibre bundles arranged parallel to the skin surface with a few fine elastic fiber bundles, which stain black with Verhoeff's elastin stain. Many fibrocytes are scattered among the collagen fibers. Branches from the main blood vessels, pigment cells and nerves in the subcutis run through this layer and supply the capillaries in the stratum laxum. In the inner surface of the stratum compactum, a layer of small-branched pigment cells is discernible. The staining pattern of this layer is similar to that of the stratum laxum.
Subcutis
This is the innermost region of the skin and lies in between the stratum compactum and the muscles. It is composed of loose connective tissues and is richly infiltrated with fat cells that in routine HE preparations appear as empty spaces. Many blood vessels and nerves may be found in this region.
Denticles. Dermal denticles (denticles) in the skin of A. dolichopterus are bilaterally symmetrical structures. They comprise various non cellular and cellular components and both epidermal and dermal elements contribute to their structure. They have caudally pointed crown and embedded broad basal tissue, which latter is narrower as the pedestal. These denticles are well seated with the narrow pedestal basal parts inserted into the cup shaped structures of the elevated mineralized osteoderms to which they are firmly anchored by the attachment fibers. Enameloid and dentin are the components of the crown (Fig. 1B, C). A thin layer of enameloid covers the dentin. The dentine layer is thicker, and thickens by continuous deposition of dentin centripetally towards the center of the pulp cavity. Odontoblasts are present along the dentine. These cells extend odontoblastic processes to form dentine tubules. The pulp cavity is supplied with the cells and some connective tissues (Fig. 1C). The dentine stains red with HE, magenta with PAS and AB-PAS (Fig. 1B). The attachment fibers appear pinkish with HE, magenta with PAS and AB-PAS and remain unstained with AB at pH 2.5 and pH 1.0.
Discussion
The skin of A. dolichopterus comprises three principal layers: the epidermis, dermis and subcutis. The epidermis of A. dolichopterus is thicker than the skin of other teleosts (Banerjee & Mittal, 1975; Park et al., 2003; Lizarazo et al., 2008) ranging two to three time fold. The primary function of the epidermis is protection against environmental hazards. In fish, mucogenic cells generally provide this function by secreting their contents on the surface, but siluroids generally present a low number of mucous cells in epidermis. In A. dolichopterus they are voluminous, but their density is remarkably low and they are confined to the upper layers of the epidermis. In Bagarius bagarius, a scaleless siluroid fish, mucous cells are few and restricted to epidermal furrows; keratinized epithelial cells and epidermal plaques provide protection (Mittal & Munshi, 1970; Mittal & Whitear, 1979; Mittal et al., 1995). Rita rita presents small mucous cells at a low density, but in contrast presents numerous club cells that are arranged in several layers (Mittal, 1968). In Barbus sophor (=Punctius sophore), in regions where mucous cells are numerous and are well developed, club cells are either sparse or absent, whereas in regions where mucous cells are smaller and present in a small number, the club cells are numerous and well developed (Mittal et al., 1976). Singh & Mittal (1990) suggested that the low density of mucous cells is compensated by the high density of club cells as an effective defense mechanism, in carps of India. However, club cells were not observed in the dorsal skin of Ancistrus. The reduced number of mucous cells and absence of club cells indicate that their protective function has been taken up by the thick epidermis and also by the osteoderms and the denticles impregnated in its dermis. Mucous cells of Ancistrus present a voluminous aspect which seems to compensate its low number resulting in a high mucogenicity that was observed trhough histochemistry.
Glycoprotein contents of epidermal secretions in fish may vary according to the species and cell types (Whitear, 1986). In A. dolicopterus, a high level of mucogenicity may be an adaptation to its peculiar bottom-scooping habitats. This species frequently deal with organic debris in the bottom when searching for food, and must keep the skin surface clean then the mucus can precipitate mud held in suspension (Hora, 1934). The secretion of copious mucus was reported in fishes which burrow and live associated with muddy waters (Mittal & Munshi, 1971; Mittal & Banerjee, 1975). The lubricant role of mucus reduces body friction in water, helping on swimming and also protecting the body from abrasion during burrowing and nest digging (Mittal & Munshi, 1971; Rosen & Cornford, 1971; Mittal et al., 1994). The detection of glycoproteins with oxidizable vicinal diols and O-sulphate esters in epithelial and mucous cells in the epidermis indicates its role on lubrication of skin surface in A. dolichopterus. Mucus can act as barrier to various pathogens and prevent their colonization in the epidermis (Lewis, 1970; Mittal et al., 1994). Furthrmore, the sulphate groups provide an acidification of glycoproteins which is effective to prevent bacterial and viral invasion (Lewis, 1970; Mittal et al., 1994).
The marked histological and biochemical similarity between fish eosinophils and mammalian mast cells has been noted by Ellis (1977, 1985) and reviewed by Powell et al. (1993b). Eosinophils release toxic proteins and oxygen radicals onto the body surface of multicellular parasites in areas of inflammation. Degranulation of eosinophils induces a fast generalized inflammation and infiltration by wondering leucocytes as well as destruction of invading parasites. A large population of eosinophilic granular cells is present in the upper epidermal layers of A. dolichopterus. There is no clear-cut distinction among various categories of granulocytes in the dorsal part of the skin. But according with their size, structure and staining these were considered as eosinophilic cells (Rombout et al., 1989; Powell et al., 1990, 1993a, 1993b; Cross & Matthews, 1991). The presence of these cells plays an important role on the protection of A. dolichopterus and other catfish species which can be exposed to a large number of skin parasites in their peculiar habitat (Thatcher, 1991).
Eosinophils in fish can be implicated in both specific immunological and general defense processes (Powell et al., 1990). They show degranulation after exposure pathogenic bacteria (Ellis, 1985; Powell et al., 1993a) and other stressors as UV light (Roberts & Bullock, 1981) and substance P (Powell et al., 1993b). The granulocytes were motile, chemotactic to histamine and to the degranulation products of neighboring granulocytes, and their population increased by handling stress within the epidermis (Barnett et al., 1996). Granulocytes contribute to wound healing in carp Cyprinus carpio (Iger & Abraham, 1990) and have been reported to be part of the trout immune response to parasitic infections (Sharp et al., 1989). The presence of metazoan parasites in species of sedentary catfishes, astroblepids, callichthyids, hypophthalmids, and loricariids, including Ancistrus from tropical (Amazon) and sub-tropical South American streams has been documented (Freihofer & Neil, 1967; Kohn & Cohen, 1998; Thatcher, 1998). Thus, the presence of EGCs in appreciable amount in the epidermis of Ancistrus can be seen as a system that destroys such multicellular parasites. This indicates a relationship between this epidermal feature and ectoparasite protection in fish.
A continuous layer of melanocytes immediately beneath the basement membrane, and their random presence in different layers of the dermis and epidermis in bottom dwelling fish impart coloration and camouflaging functions. Toledo & Jared (1993), while working with amphibian skin, suggested that the dermal chromatophore units provide patterns of coloration and may also function to absorb or reflect radiations (thereby contributing to regulation of body temperature). They may play similar roles in Ancistrus too, since this air-breathing fish can be exposed to high solar radiation levels during episodes of environmental hypoxia and dry season in the Amazon (Val & Almeida-Val, 1995).
Taste buds are characteristic features of fish skin and assist them in the recognition and location of food. In many ostariophysans (e.g., cyprinids and catfish) external taste buds cover the entire body surface and aggregate in areas that frequently contact and locate food, such as lips, barbells or elongated fins (Kotrschal, 1996). A low number of taste buds in the dorsal skin was expected in A. dolichopterus and can be seen as an adaptive response because these algal grazers feed by night and bear long branched-tentacles on the snout, which may have sufficient taste buds. However, it needs detailed investigation.
In A. dolichopterus the stratum compactum of the dorsal part of the skin is very thin. But the stratum laxum, unlike that of other siluroid fishes, Clarias batrachus (Banerjee & Mital, 1975), R. rita (Mittal, 1968), and B. bagarius (Mittal & Munshi, 1970) is very thick, provided with the osteoderms, and is distinct from the stratum compactum. The thickness of this layer is increased in fishes that present well developed scales and comparatively thinner in species that have less developed scales. In scaly fishes, the scales are lodged in the characteristic connective tissue pockets in the stratum laxum. Thus a well developed stratum laxum is found in fishes having highly developed scales or osteoderms may be explained. In scaleless fishes, there is no need of such structural modifications and then stratum laxum remains insignificant. Banerjee & Mittal (1975) suggested an inverse relationship between the thickness of the stratum laxum and the stratum compactum in fishes. This corroborates our view regarding Ancistrus. The thick integument together with denticles and osteoderms helps to keep them at the bottom. The compact dermis of the Ancistrus thus evidently serves two functions: it offers effective protection and, because of its weight, facilitates precise maneuvering near the bottom.
A subcutis is present in all the fishes except Amia calva (Rabl, 1931). But Bhatti (1938) did not describe this layer while working with the integument of a number of siluroids. As observed in A. dolichopterus, subcutis is also poorly developed in B. bagarius (Mittal & Munshi, 1970), thin and inconspicuous in Mestacembelus and Amphinuous (Mittal & Munshi, 1971) but well differentiated in Heteropneustes and Clarias (Mittal & Munshi, 1971; Banerjee & Mittal, 1975), respectively.
Denticles cover the head and body of Ancistrus. These sharp, conical, backwardly-directed denticles comprise a dentine cone covered by a hypermineralized cap that surrounds the pulp cavity which is supplied with the cells and connective tissue, are similar to the placoid scales and are the true homologous to true teeth. Bhatti (1938) suggested these denticles to be homologous with true teeth. In Corydoras aeneus (Sire & Huysseune, 1996) and Denticeps clupeoides (Sire et al., 1998) the denticles present in the skin are true odontodes. This structural similarity between the denticles and the elasmobranch placoid scales shows that a morphogenetic/histogenetic potential has been present in vertebrate skin even since the early times of Agnathan evolution and that such structures were expressed convergently in various groups during fish evolution. The resemblance of the cell types in the epidermis of elasmobranches as Raja to those known from teleost fishes, also support this idea (Whitear & Moate, 1998).
A conspicuous feature such as loricariid denticles raises the question of their biological significance. They provide effective protection from abrasion and deter enemies. These crevice dwellers use their spines to jam themselves into crevices when any attempt is made to remove them, and the movable hooked spines on the cheek skin of Ancistrus are also used in this way. Thus dermal bony plates and denticles of Ancistrus are noteworthy ostariophysan innovations that facilitate the invasion of new habitats or niches.
Acknowedgements
TKG was supported by a visiting fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasil). Authors thank the Departmento de Histologia, Universidade Federal do Amazonas for providing the laboratory facilities and Silvio Manfredo Vieira and Maria de Nazaré Paula da Silva for technical assistance in photography. ALV and VMFAV are recipients of research fellowships from CNPq.
Literature Cited
Accepted September 4, 2010
Published December 16, 2010
References
- Bancroft, J. D. & A. Stevens. 1982. Theory and practice of histological techniques. Edinburgh, Churchill Livingstone, 663p.
- Banerjee, T. K. & A. K. Mittal. 1975. Histochemistry and the functional organization of the skin of a "live-fish" Clarias batrachus (Lin.) (Clariidae, Pisces). Mikroskopie, 31: 333-349.
- Barnett, R. R., T. Akindele, C. Orte & K. L. Shephard. 1996. Eosinophilic granulocytes in the epidermis of Oreochromis mossambicus gill filaments studied in situ Journal of Fish Biology, 49: 148-156.
- Bhatti, H. K. 1938. The integument and dermal skeleton of Siluroidea. Transactions of the Zoological Society of London, 24: 1-102.
- Burgess, W. E. 1989. An atlas of freshwater and marine catfishes. A preliminary survey of the siluriformes. Neptune City, T. F. H. Publications, 784p.
- Cross, M. L. & R. A. Matthews. 1991. Identification of a new granulocyte type in the skin of carp Cyprinus carpio (L). Journal of Fish Biology, 39: 279-283.
- Ellis, A. E. 1977. Leukocytes of fish - review. Journal of Fish Biology, 11: 453-491.
- Ellis, A. E. 1985. Eosinophylic granular cells (EGC) and histamine responses to Aeromonas salmonicida toxins in rainbow trout. Developmental and Comparative Immunology, 9: 251-260.
- Elsner, J. & A. Kapp. 2000. Activation and modulation of eosinophils by chemokines. Allergologie, 23: 59-72.
- Ferencik, M. 1993. Handbook of Immunochemistry. London, Chapman and Hall, 387p.
- Freihofer, W. C. & E. H. Neil. 1967. Commensalism between midge larvae (Diptera: Chironomidae) and catfishes of the families Astroblepidae and Loricariidae. Copeia, 1: 39-45.
- Hora, S. L. 1934. A note on the biology of the precipitating reaction of the mucus of boro fish Pisodonophis boro (Ham. Buch.). Journal and Proceedings of the Asiatic Society of Bengal, 29: 271-274.
- Iger, Y. & M. Abraham. 1990. The process of skin healing in experimentally wounded carp. Journal of Fish Biology, 36: 421-437.
- Imaki, H. & W. Chavin. 1984. Ultrastructure of mucous cells in the sarcopterygian integument. Scanning Electron Microscopy, 1: 409-422.
- Isbrücker, I. J. H. 1981. Classification and catalogue of the mailed Loricariidae (Pisces, Siluriformes). Verslagen en technische gegevens. Universiteit van Amsterdam, n 22, 181p.
- Lizarazo, R. J. B., M. Q. Virguez, E. G. Ramírez, D. R. Caicedo, H. H. Giraldo. 2008. Histología y morfometría de piel del pez Eremophilus mutisii (Trichomycteridae, Siluriformes). Revista de Biologia Tropical, 56(2): 885-893.
- Kohn, A. & S. C. Cohen. 1998. South American Monogenea list of species, hosts and geographical distribution. International Journal of Parasitology, 28: 1517-1554.
- Kotrschal, K. 1996. Solitary chemosensory cells: Why do primary aquatic vertebrates need another taste system? Trends in Ecology and Evolution, 11: 110-114.
- Lewis, R.W. 1970. Fish cutaneous mucus: a new source of skin surface lipids. Lipids, 5: 947-949.
- Lowe-McConnell, R. H. 1975. Fish Communities in Tropical Freshwaters - Their distribution, ecology and evolution. London, Longman Inc, 283p.
- Mittal, A. K. 1968. Studies on the structure of the skin of Rita rita (Ham.) (Bagridae, Pisces) in relation to its age and regional variations. Indian Journal of Zoology, 9: 61-78.
- Mittal, A.K., S. K. Agarwal & T. K. Banerjee. 1976. Protein and carbohydrate histochemistry in relation to the keratinization in the epidermis of Barbus sophor (Cyprinidae, Pisces). Journal of Zoology, 179: 1-17.
- Mittal, A. K. & T. K. Banerjee. 1975. Histochemistry and structure of skin of a murrel, Channa striata (Bloch, 1797) (Channiformes, Channidae). 1. Epidermis. Canadian Journal of Zoology, 53: 833-843.
- Mittal, A. K., T. K. Garg & M. Verma. 1995. Surface architecture of the skin of the Indian catfish, Bagarius bagarius (Hamilton) (Sisoridae; Siluriformes). Japan Journal of Ichthyology, 42: 187-191.
- Mittal, A. K. & J. S. D. Munshi. 1970. Structure of the integument of a fresh-water teleost, Bagarius bagarius (Ham.) (Sisoridae, Pisces). Journal of Morphology, 130: 3-10.
- Mittal, A. K. & J. S. D. Munshi. 1971. A comparative study of the structure of the skin of certain air-breathing fresh-water teleosts. Journal of Zoology, 163: 515-532.
- Mittal, A. K. & M. Whitear. 1978. A note on cold anaesthesia of poikilotherms. Journal of Fish Biology, 13: 519-520.
- Mittal, A.K. & M. Whitear. 1979. Keratinization of fish skin with special reference to the catfish Bagarius bagarius Cell and Tissue Research, 202: 213-230.
- Mittal, A. K., T. Ueda, O. Fujimori & K. Yamada. 1994. Histochemical analysis of glycoproteins in the unicellular glands in the epidermis of an Indian fresh water fish Mastacembelus panculus (Hamilton). Histochemistry Journal, 26: 666-677.
- Park, J.-Y., I.-S. Kim & S.-Y Kim. 2003. Structure and histochemistry of the skin of a torrent catfish, Liobagrus mediadiposalis Environmental Biology of Fishes, 66(1): 3-8.
- Pearse, A. G. E. 1985. Histochemistry, theoretical and applied. 2. Analytical technology, London, Churchill Livingstone, 1055p.
- de Pinna, M. C. C. 2006. Diversity of Tropical fishes. Pp. 47-84. In: Val, A. L., V. M. F. Almeida-Val & D. J. Randall (Eds.). Physiology of Tropical Fishes. London, Elsevier, 634p.
- Powell, M. D., H. A. Briand, G. M. Wright & J. F. Burka. 1993a. Rainbow trout (Oncorhynchus mykiss Walbaum) intestinal eosinophilic granule cell (EGC) response to Aeromonas salmonicida and Vibrio anguillarum exteracellular products. Fish and Shell Fish Immunology, 3: 279-289.
- Powell, M. D., G. M. Wright & J. F. Burka. 1990. Eosinophilic granular cells in the gills of rainbow trout, Oncorhynchus mykiss: Evidence of migration? Journal of Fish Biology, 37: 495-497.
- Powell, M. D., G. M. Wright & J. F. Burka. 1993b. Morphological and distributional changes in the eosinophilic granular cells (EGC) population of the rainbow trout (Oncorhynchus mykiss Walbaum) intestine following systemic administration of capsacin and substance P. Journal of Experimental Zoology, 266: 19-30.
- Rabl, H. 1931. Integument der Anamnier. Pp. 271-306. In: Bolk, L., E., E. Goppert, E. Kallius & W. Lubosch (Eds.). Handbuch der verleichenden Anatomie der Wirbeltiere 1, Berlin, Urban and Schwarzenberg, 446p.
- Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. 2003. Check list of the freshwater fishes of South and Central America. Porto Alegre, Edipucrs, 729p.
- Roberts, R. J. & A. M. Bullock. 1981. Recent observations on the pathological effect of ultraviolet light on fish skin. Fish Pathology, 15: 237-239.
- Rombout, J. H. W. M., H. E. Bot & J. J. Taverne-Thiele. 1989. Immunological importance of the second gut segment of carp. II. Characterisation of mucosal leucocytes. Journal of Fish Biology, 35: 167-178.
- Rosen, M. W. & N. E. Cornford. 1971. Fluid friction of fish slimes. Nature, 234: 49-51.
- Sharp, G. J. E., A. W. Pike & C. J. Secombes. 1989. The immune response of wild rainbow trout, Salmo gairdneri Richardson to naturally acquired plerocercoid injections of Diphyllobothrium dendriticum (Nitzsch 1824) and D ditremum (Creplin 1825). Journal of Fish Biology, 35: 781-794.
- Singh, S. K. & A. K. Mittal. 1990. A comparative study of the epidermis of the common carp and the three Indian major carp. Journal of Fish Biology, 36: 9-19.
- Sire, J. Y. & A. Huysseune. 1996. Structure and development of the odontodes in an armoured catfish, Corydoras aeneus (Siluriformes, Callichthyidae). Acta Zoologica, 77: 51-72.
- Sire, J. Y., S. Marin & F. Allizard. 1998. Comparison of teeth and dermal denticles (Odontodes) in the teleost Denticeps clupeoides (Clupeomorpha). Journal of Morphology, 237: 237-255.
- Sire, J. Y. & F. J. Meunier. 1993. Superficial ornamentation and structure of the osseous dermal plates in some armored Siluriforms (Loricariidae, Callichthyidae, Doradidae). Annales Des Sciences Naturelles-zoologie et Biologie Animale, 14: 101-123.
- Thatcher, V. E. 1998. Copepods and fishes in the Brazilian Amazon. Journal of Marine Systems, 15: 97-112.
- Toledo, R. C. & C. Jared. 1993. Cutaneous adaptations to water balance in amphibians. Comparative Biochemistry and Physiology, 105: 593-608.
- Val, A. L. & V. M. F. Almeida-Val, 1995. Fishes of the Amazon and their environments. Physiological and Biochemical Features. Berlin, Springer-Verlag , 224p.
- Whitear, M. 1986. Epidermis. Pp. 8-38. In: Bereiter-Hahn, J., A. G. Matoltsy & K. S. Richards (Eds.). Biology of the Integument, Vertebrates. Berlin, Springer-Verlag, 870p.
- Whitear, M. & R. Moate. 1998. Cellular diversity in the epidermis of Raja clavata (Chondrichthyes). Journal of Zoology, 246: 275-285.
Publication Dates
-
Publication in this collection
06 Jan 2011 -
Date of issue
2010
History
-
Received
04 Sept 2010 -
Accepted
16 Dec 2010

 Histochemistry and functional organization of the dorsal skin of Ancistrus dolichopterus (Siluriformes: Loricariidae)
Histochemistry and functional organization of the dorsal skin of Ancistrus dolichopterus (Siluriformes: Loricariidae)