Abstracts
Recent years have witnessed a substantial increase in the number of seric determinations of vitamin D, in aworldwide basis. At Hospital das Clínicas of Faculdade de Medicina of Universidade de São Paulo that increase reached 700% over the last four years. Nevertheless there are many controversies on the literature about the role of vitamin D in conditions unrelated to themusculoskeletal system. In this study the metabolism, sources and actions of vitamin D on the body are reviewed. Observational studies, clinical trials, systematic reviews and metanalysis which focused on the relationship between the vitamin and conditions such as cancer, cardiovascular disease, diabetes and falls were searched on the literature, analyzed and discussed. Results are presented as quiz and answer, tables and a figure. The role of vitamin D on the above-mentioned conditions is discussed, and the controversial issues stressed.
Vitamin D; Cardiovascular diseases; Cancer; Diabetes; Falls; Review
O número de dosagens do nível sérico de vitamina D tem apresentado crescimento muito expressivo nos últimos anos em todo o mundo. No Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo houve aumento de cerca de 700% em quatro anos nas solicitações desse hormônio. No entanto, há controvérsias na literatura sobre a real utilidade de sua dosagem e/ou suplementação, exceto em situações diretamente relacionadas ao metabolismo ósseo. No presente trabalho são revistos o metabolismo, as fontes e as ações da vitamina D no organismo. Estudos observacionais, ensaios clínicos, revisões sistemáticas e metanálises, cujo foco é a relação entre vitamina D e doenças ou condições clínicas, como câncer, doenças cardiovasculares, diabetes e quedas, foram pesquisados na literatura, analisados e discutidos. Os resultados estão apresentados em forma de perguntas e respostas, tabelas e figura. Discute-se o papel da vitamina D em todas essas situações, e salientam-se os pontos controvertidos.
Vitamina D; Doenças cardiovasculares; Câncer; Diabetes; Quedas; Revisão
REVIEW ARTICLE
IGeneral Practice and Propedeutics Service, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil
IICentral Laboratory Division - LIM03, HC-FMUSP, São Paulo, SP, Brazil
IIIClinical Emergency Service, HC-FMUSP, São Paulo, SP, Brazil
IVImmunology Service, HC-FMUSP, São Paulo, SP, Brazil
ABSTRACT
Recent years have witnessed a substantial increase in the number of seric determinations of vitamin D, in a worldwide basis. At Hospital das Clínicas of Faculdade de Medicina of Universidade de São Paulo that increase reached 700% over the last four years. Nevertheless there are many controversies on the literature about the role of vitamin D in conditions unrelated to the musculoskeletal system. In this study the metabolism, sources and actions of vitamin D on the body are reviewed. Observational studies, clinical trials, systematic reviews and metanalysis which focused on the relationship between the vitamin and conditions such as cancer, cardiovascular disease, diabetes and falls were searched on the literature, analyzed and discussed. Results are presented as quiz and answer, tables and a figure. The role of vitamin D on the above-mentioned conditions is discussed, and the controversial issues stressed.
Keywords: Vitamin D; Cardiovascular diseases; Câncer; Diabetes; Falls; Review
Introduction
Context
The number of ordered laboratory tests has been rising steadily worldwide, among them, vitamin D measurement. The number of such analyses in the Hospital das Clínicas of the Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP) increased from 6,810 in 2007 to 52,997 in 2011, with a further increase of 60% in 2012, which occurred without an equivalent increase number of patients attended to in the same period. This is probably caused by the recent exponential increase in the number of published studies in medical literature addressing the possible role of vitamin D in different clinical situations, not only those related to bone metabolism.1 Studies conducted in the United States demonstrated that three quarters of the White population and 90% of individuals of Black, Hispanic, and Asian descent in the US have low blood levels of vitamin D.
It is estimated that over 1 billion people worldwide have low vitamin D levels,2 which appears to constitute an actual "epidemic" of hypovitaminosis D, with possible serious consequences for public health. Alternatively, it can be argued that low serum levels of vitamin D may merely be an indicator of unhealthy life habits or poor health, and, except in cases of rickets and osteomalacia, they may not necessarily be involved in the cause of other diseases.
The present study reviewed the physiology of vitamin D and its measurement methods, and critically evaluated the latest evidence on some of the possible extra-osseous effects of vitamin D as shown by clinical trials, systematic reviews, meta-analyses, and traditional reviews in the literature, as well as the recommendations of different international societies for its measurement and supplementation.
Sources of vitamin D
The main forms of biological vitamin D or calciferol (the group of chemical substances related to vitamin D) in nature and the human body are shown in Table 1.
The need for vitamin D is 600 IU/day for individuals aged 1 to 70 years, and 800 IU/daily for those older than 70 years, which results in serum levels > 20 ng/mL, provided there is a minimum level of sun exposure.
Serum levels of vitamin D are influenced by several factors, such as obesity, sun exposure, physical activity, nutritional status, skin pigmentation, and medications. Patients who have undergone bariatric surgery and patients with chronic renal failure have a higher risk of vitamin D deficiency. Black individuals require a three to five times longer sun exposure than whites to produce the same amount of vitamin D. The use of sun protection factor 30 sunscreen reduces vitamin D production by over 95%. Anticonvulsants and antiretroviral drugs accelerate the catabolism of vitamin D. Endogenous sources of vitamin D last twice as long in the body than exogenous ones.2,3
The following presents the amount of vitamin D in each food:
Metabolism
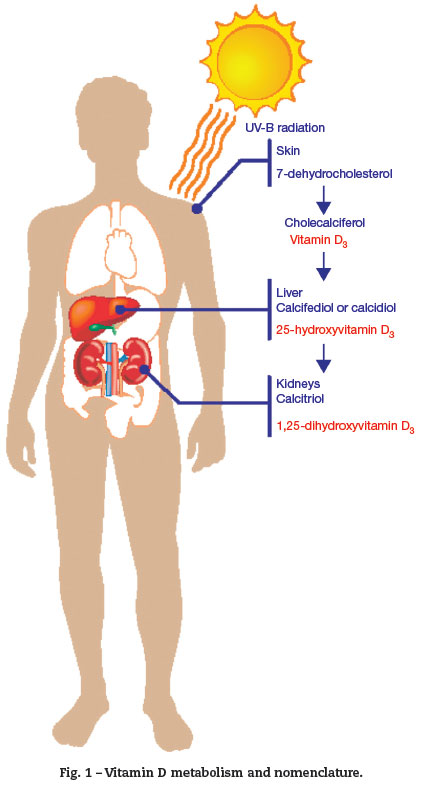
The major source of vitamin D is the epidermis. Vitamin D3 is produced in the skin by a reaction mediated by ultraviolet B (UVB) radiation, whose wavelength is in the range of 290 to 315 nm. This is a photolytic reaction, not enzymatic, and converts 7-dehydrocholesterol into pre-vitamin D3 . Pre-vitamin D3 undergoes another non-enzymatic reaction, which produces a thermal isomerization in the skin, reaching a vitamin D peak in 30 to 60 days after sun exposure. From the skin, vitamin D3 enters the circulation and reaches the liver, where the P450-family enzymes convert it into 25-hydroxyvitamin-D3 or 25(OH)D3 (calcidiol). 25(OH)D3 binds to serum proteins, remaining as the most stable metabolite of vitamin D; its measurement is the most suitable test for assessing body status. It is related to the dermal synthesis and ingestion.4
25(OH)D3 is converted into 1,25-dihydroxyvitamin D or 1,25(2OH)D3 (calcitriol) by the mitochondrial enzyme CYP27B1-hydroxylase of the epithelial cells in the proximal renal tubules (Fig. 1). 1,25(2OH)D3 binds to high-affinity tissue receptors, modulating gene expression and subsequent actions. Its concentration is approximately 0.1% of that of 25(OH) D3 prohormone. Its synthesis is stimulated by the parathyroid hormone (PTH) and inhibited by the fibroblast growth factor 23(FGF23), produced in osteocytes. Thus, a decrease in 25(OH)D3 stimulates PTH production.
In the intestine, vitamin D stimulates calcium and phosphorus absorption. Without vitamin D, only 10% to 15% of the calcium and 60% of the phosphorus in the diet is absorbed.2 At sufficient levels, vitamin D increases the absorption of calcium to 30% to 40% and the absorption of phosphorus to 80%. 1,25(2OH)D3 binds to specific receptors (vitamin D receptors [VDR]) of osteoblasts, stimulating RANK-ligand expression. The latter interacts with the receptor activator of nuclear factor kappa-b, which induces the immature monocytes to develop into mature osteoclasts, which in turn release the stores of calcium from bones. Furthermore, from the evolutionary point of view, vitamin D produces a cytokine that protects the cell from microbial invasion. This cytokine is produced by monocytes/macrophages, and has an intracellular action.5
Methods of laboratory measurement and reference values
The measurement of serum 25(OH)D3 is useful in the evaluation of the body load of vitamin D, as well as in the differential diagnosis of hypercalcemia (which includes exogenous poisoning). The measurement of serum 1,25(2OH)D3 is indicated in chronic renal failure, hypercalcemia associated with granulomatous diseases, and vitamin D-dependent rickets type I.
Currently, the ideal methods for measuring 25(OH)D3 are based on high-performance liquid chromatography (HPLC) or liquid chromatography with tandem mass spectrometry detection (LC-MS/MS). In practice, however, automated immunoassays are the methods most commonly used by clinical laboratories. This method simultaneously measures 25(OH)D2 and 25(OH)D3 . HPLC and mass spectrometry are capable of differentiating between these two forms, providing distinct results for each of the fractions.6
The optimal serum level of 25(OH)D3 is yet to have a consensus in the literature. In theory, the optimal level of vitamin D would be that required to maintain adequate levels of PTH. The decrease in calcium absorption by the intestine causes a decrease in plasma calcium levels, which activates calcium-sensing receptors in the parathyroid membrane, releasing PTH and increasing PTH gene expression.7
The interaction of PTH with PTH receptor/PTHrP of the epithelial cell membrane of the renal tubules leads to an increase in CYP2/B1 gene. This converts 25(OH)D3 into 1,25(2OH)D3 . This vitamin binds to serum proteins, reaching the cells containing vitamin D receptors. In the intestine, they promote calcium and phosphorus absorption, and in the bone, they release calcium and phosphorus from the mineral matrix. When there is normalization of serum calcium levels, FGF23is released by the bones, interrupting the process.8
This knowledge led to the improvement of the definition of vitamin D insufficiency and deficiency in terms of the increase of immunoreactive serum PTH (iPTH). iPTH increases when the serum levels of 25(OH)D3 decrease to less than 30 ng/mL or 75 nmol/L.
The American Society of Endocrinology suggests the following reference values for 25(OH)D3:
Deficiency: < 20 ng/mL
Insufficiency: 21 to 29 ng/mL
Optimal: > 30 ng/mL
Skeletal abnormalities are observed only in cases of vitamin D deficiency. In the U.S., 25% to 35% of the female population is included in this group. There are some plausible explanations for this fact: decrease in milk consumption (fortified with vitamin D), use of sunscreens, decreased sun exposure, and increased body mass index of the U.S. population.8
Methods
Systematic and traditional literature reviews, metaanalyses, and major clinical trials regarding vitamin D measurement and/or supplementation and the possible association with cancer, cardiovascular disease (CVD), diabetes, and falls were searched in the MEDLINE, PubMed, and SciELO databases.
This literature search identified articles submitted to critical evaluation, taking the following questions as common methodological denominators for each type of clinical situation:
Is there is any biological plausibility regarding the hypothesis that vitamin D is associated with the clinical situation?
Is there any epidemiological evidence associating serum vitamin D levels with the clinical situation in humans?
Are there systematic reviews of these studies on the asso ciation of vitamin D with the clinical situation in humans?
Is this evidence conclusive in itself?
Are there any randomized controlled trials (RCTs) evaluating the association of vitamin D supplementation with morbidity and mortality?
Can it be concluded that vitamin D is associated with morbidity and mortality and that its replacement can prevent them?
After analyzing all the specific literature, the consolidated findings were grouped by clinical situations, and then the findings were homogenized to allow for the presentation of results and drawing of conclusions.
Results
The answers to the analyzed clinical situations are detailed below, and summarized in Table 2.
Cancer
Is there is any biological plausibility regarding the hypothesis that vitamin D is associated with cancer?
Yes. Studies in cell cultures and experimental animal models suggest that 1,25(2OH)D3 (calcitriol) promotes cell differentiation, inhibits vascular and cancer cell proliferation, and also presents anti-inflammatory and proapoptotic properties.9
Is there any epidemiological evidence associating serum vitamin D levels with cancer in humans?
Yes. Ecological correlations (e.g., increased incidence of cancer in countries with less sun exposure, higher cancer mortality in the winter and among black individuals) and some isolated observational studies (cohort and case-control) associated low serum 25(OH)D3 levels with higher cancer incidence and mortality, although other studies have not confirmed this association.10
Are there systematic reviews of these studies on the association of vitamin D with cancer in humans?
Yes. Meta-analyses of observational studies have suggested a possible association between higher levels of vitamin D and a slight reduction in the incidence of colorectal cancer, increased mortality from overall cancer (only in men), and no association with prostate and breast cancer.10 The pooled analysis of ten cohort studies included in the "Vitamin D pooling project" also found no significant association of vitamin D with less common cancers (endometrial, esophageal, stomach, kidney, non-Hodgkin lymphoma, ovarian, and pancreatic). On the contrary, a higher risk of pancreatic cancer was associated with serum concentrations of vitamin D > 40 ng/mL.11
Is this evidence conclusive in itself?
No. The ambiguity of the results obtained from observational studies may be due to confounding factors, such as obesity (adipose tissue sequesters vitamin D), sedentary lifestyle (little sun exposure), black skin (decreased production of vitamin D, even with sun exposure), and type of diet (low vitamin intake). Furthermore, these studies are subject to reverse bias, that is, the person with cancer tends to be more reclusive, to decrease sun exposure, to be undernourished, and thus to have lower serum levels of vitamin D.
Are there any RCTs evaluating the association of vitamin D supplementation with cancer incidence and mortality?
Yes. Although, to date, no RCTs evaluating the supplementation of vitamin D and cancer incidence and mortality as primary outcomes have been finalized, some RCTs have allowed for the analysis of both as secondary outcomes. In the largest of them, the Women's Health Initiative (WHI), which followed 36,282 women between 50 and 79 years for seven years, divided into two groups (placebo vs. 400 IU vitamin D/day + calcium), there was no difference either in cancer incidence or mortality. [12] Similar results were observed in two other RCTs developed in Oxford, UK, and in Nebraska, USA; however, in the latter, the comparison of two study subgroups (calcium vs. 1,000 IU vitamin D/day + calcium) showed a significant reduction in overall cancer incidence and mortality.9,10
Can it be concluded that low vitamin D levels are associated with higher cancer incidence and mortality and that its replacement can prevent it?
No. In light of the best available scientific evidence, it is premature to consider that lower serum levels of vitamin D may, by themselves, increase cancer incidence or mortality, and justify supplementation for disease prevention.12 To support good medical recommendations, other RCTs are still needed, with more diverse target populations, higher dose vitamin D supplementation (> 800 IU/day), longer duration of follow-up in the study groups, and with cancer incidence and mortality as primary outcomes.
Cardiovascular diseases
Is there is any biological plausibility regarding the hypothesis that vitamin D is associated with CVD?
Yes. Experimental laboratory studies have suggested possible mechanisms of action or influence of vitamin D on the cardiovascular system. For example, vitamin D deficiency activates the renin-angiotensin-aldosterone system, which can lead to systemic arterial hypertension (SAH) and left ventricular (LV) hypertrophy. Another consequence of deficiency is the increase in PTH, which leads to increased insulin resistance, diabetes mellitus type 2, SAH, and inflammation. Vitamin D inhibits the proliferation of cardiomyoblasts by promoting cell cycle arrest, and increases cardiomyotubule formation, without inducing apoptosis. It has also been observed that vitamin D attenuates LV dysfunction in animal models and humans.
Is there any epidemiological evidence associating serum vitamin D levels with CVD in humans?
Yes. Cross-sectional studies have raised the suspicion of this association (e.g., the incidence of cardiovascular disease is higher in population groups with lower levels of vitamin D, and vice-versa). A Korean survey conducted in 2008-2009 demonstrated an association between lower levels of vitamin D and higher CVD mortality.13 Isolated observational studies have shown an association between lower serum levels of 25(OH)D3 and increased CVD incidence and/or mortality, including SAH and coronary heart disease, although there are other studies that did not show this association.
Are there systematic reviews of these studies on the association of vitamin D with CVD in humans?
Yes. Regarding SAH, a meta-analysis of three cohort studies, which totaled 32,181 individuals followed-up between seven and ten years, showed increased risk among individuals with low blood levels of vitamin D. A systematic review of seven cohorts (n = 43,527; five to 27 years of follow-up) demonstrated a trend of inverse correlation between vitamin D levels and CVD incidence and mortality, including coronary heart and cerebrovascular disease.[14] In addition to these, part of the Tromso study was recently published, which longitudinally evaluated the association between the presence of genetic polymorphisms related to vitamin D and a) outcomes (myocardial infarction and mortality), b) risk factors for CVD; no consistent association was identified.15
Is this evidence conclusive in itself?
No. The evidence is insufficient for the same reasons described for cancer, especially regarding the methodological limitations inherent to observational studies, which cannot be used to demonstrate the cause-effect association between variables, only possible associations.
Are there any RCTs evaluating the association of vitamin D supplementation with CVD incidence and mortality?
Yes. A meta-analysis of clinical trials that included 70,528 individuals with a median age of 70 years (86.8% women), whose intervention groups received varying doses of vitamin D, alone or in combination with calcium, demonstrated a borderline reduction in mortality in individuals receiving vitamin D + calcium (RR = 0.91, 95% CI = 0.84 to 0.98) and no statistically significant effect on those receiving vitamin D only.16 Another RCT, in which C-reactive protein (CRP) levels and arterial blood flows were measured in 114 women who received 25,000 IU/day of vitamin D3 or placebo showed no difference between the groups after four months of follow-up.17 In addition to these studies, a meta-analysis of ten clinical trials showed no effect of vitamin D on systolic or diastolic blood pressure levels, and four other RCTs showed no superior effect of vitamin D over placebo on the incidence of myocardial infarction, angina pectoris, stroke, and transient ischemic attack.14
Can it be concluded that vitamin D is associated with higher CVD incidence and mortality and that its replacement can prevent it?
No. The available RCTs demonstrated no consistent causal association between vitamin D supplementation and reduced overall CVD incidence or mortality, or from hypertension or ischemic heart disease alone. Other ongoing RCTs may provide more information.
Diabetes mellitus
Is there is any biological plausibility regarding the hypothesis that vitamin D is associated with diabetes?
Yes. Several studies indicate the possibility of using vitamin D in diabetes mellitus prevention and treatment. Its actions on the immune system, for instance, could be useful in patients with diabetes mellitus type 1; vitamin D can improve the activity of beta cells, either directly through their receptors, or indirectly by regulating calcium homeostasis. It can also affect insulin sensitivity.18 There is one study that demonstrated the worsening of diabetes control, in countries with temperate climate, during winter.19
Is there any epidemiological evidence, including systematic reviews, associating serum vitamin D levels with diabetes in humans?
Yes. A cross-sectional study of the National Health and Nutrition Survey (NHANES) in the United States, which included 9,773participants, observed that the levels of 25(OH)D3 were inversely associated with the prevalence of diabetes mellitus type 2, which persisted even after controlling for other variables.20
Conversely, although in the systematic review/metaanalysis by Parker et al.21 the combined results demonstrated a decrease in the prevalence of diabetes mellitus type 2 associated with higher levels of vitamin D, when compared with lower levels (OR 0.54, 95% CI = 0.23to 1.27), the result was only significant when the data on Black ethnicity were removed (OR = -0.36, 95% CI = 0.16 to 0.80). One of the studies included in this review showed a direct association between high levels of vitamin D and an increased prevalence of diabetes in Black individuals. Another meta-analysis in 2007 found an inverse association of the incidence of diabetes mellitus type 2 when comparing groups with higher and lower combined intake of vitamin D and calcium18 (OR = 0.82, 95% CI = 0.72 to 0.93).
Is this evidence conclusive in itself?
No. The occurrence of confounders, always possible in observational studies, makes it necessary to conduct experimental studies such as RCTs in order to establish a causal relationship between vitamin D and several diseases, including diabetes. Moreover, there are alternative explanations for the findings in these studies.
Are there any RCTs evaluating the association of vitamin D supplementation with diabetes?
Yes. In the meta-analysis and systematic review by Pittas,18 previously mentioned, interventional studies were analyzed. There were four small, short-duration studies and two of long-duration, all of which were controlled. Only one study, including 20 patients with diabetes mellitus type 2, had a favorable outcome with vitamin D use, showing improvement in insulin and C-peptide secretion in newly diagnosed patients, suggesting that vitamin D may slow the progression to clinical disease. Considered together, the studies had a limited number of participants, were predominantly short-duration, used different doses of vitamin D and calcium, and included post hoc analysis. The authors concluded for the absence of effects in healthy participants and suggested potential usefulness of vitamin D and calcium when there is no prior glucose intolerance.
Another meta-analysis included eight studies that evaluated the effect of vitamin D on blood glucose. The duration ranged from seven months to two years, and doses of vitamin D varied from 400 to 5,714 IU/day. Only two trials were considered of good quality. In five experiments in which glucose was normal at baseline, there was no effect on blood glucose or incidence of diabetes. An analysis of subgroups with inadequate blood glucose at baseline, and whose patients received 700 IU/day of D3 and 500 mg/day of calcium, showed a decrease in the tendency to worsened glycemic control, which usually occurs in these groups. In two experiments in which participants had stable disease, there were no changes after eight and 24 weeks of supplementation.14
The Women's Health Initiative (WHI) study22 is noteworthy as it was the largest on vitamin D supplementation, but the participants also received calcium: there was no statistically significant effect for any of the cardiometabolic effects assessed. It included 33,591 women with no history of diabetes; intervention consisted of supplementation with 1,000 mg of calcium and 400 IU of 25(OH)D3 /day; the outcome was new cases of diabetes. The results after a median follow-up of seven years demonstrated a relative risk of 1.01 (95% CI = 0.94 to 1.10), that is, no risk reduction. The result persisted in subgroup analyses, when noncompliance was considered and when analyzing only changes in laboratory measures.
Can it be concluded that low vitamin D levels are associated with higher diabetes incidence and mortality or that its replacement can prevent them?
There are no studies on mortality. According to this latest revision by Pittas,14 clinical trials did not confirm the cross-sectional studies that showed a consistent association between low vitamin D levels and cardiometabolic disorders.
The WHI22 study (that included calcium administration), the RCT with the highest number of participants and time of follow-up that concluded there was no association, may, however, be criticized regarding the following aspects: use of low-dose vitamin D (400 IU), difficulty of adherence throughout seven years, and allowing the use of supplements in both groups (reducing differences). Furthermore, the dose of vitamin D employed was too low to lead to a significant increase in serum levels.
Due to these criticisms, the study by Hurst,23 published in 2009, deserves a separate comment: 42 South-Asian women, aged between 23and 67 years, were treated with 4,000 IU of 25(OH)D3 /day and 39 received placebo. At the beginning they had insulin resistance (measured by HOMA1) and vitamin D < 20 ng/mL. With supplementation, vitamin D increased from 8.4 (4.4 -16.0) to 32 ng/mL (26.8-37.6) after six months.
The result showed decreased insulin resistance when 25(OH) D3 reached levels of 32 ng/mL or more. This was the first RCT that used enough supplementation to increase vitamin D to these levels, requiring doses of 2,000 IU/day or more when basal level was < 5 ng/mL. Although no clinical outcomes were studied, the authors argued that in previous studies, supplementation was not effective, either because the doses were lower, or duration was shorter (the authors did not observe significant results before six months). Based on bone markers and PTH levels, the authors suggested daily administration, but spaced doses can have better effect on adherence. They also stated that replacement would be preferable to sun exposure, due to the risk of overexposure and interpersonal variation of the needs. They finally warn about the critical role of dose sufficiency, and the need to verify the safety and effectiveness of supplementation with high doses over the long term.
Falls
Is there is any biological plausibility regarding the hypothesis that vitamin D is associated with falls?
Yes. Several studies have correlated vitamin D supplementation with reduced fractures. This decrease could be at least partly due to the fact that people fall less often.24
The possible association between falls and vitamin D deficiency is based on some findings: a) there are receptors for 1,25(2OH)D3 in muscles25,26; b) vitamin D is associated with muscle protein synthesis27,28; and c) some studies demonstrated that vitamin D improves muscle function and may reduce falls, especially when associated with calcium.29,30
There are also studies that show that PTH induces muscle catabolism, that is, when there is vless of muscle injury.
Is there any evidence associating serum vitamin D levels with falls in huitamin D deficiency, the increase in this hormone would damage muscle. Another hypothesis is decreased reflexes in individuals with vitamin D deficiency, explaining falls regardmans?
Yes, but the results are conflicting. Vitamin D deficiency in individuals aged > 65 years occurs in 40% to 50% of individuals who have no history of falls, and in 70% of those with a tendency to repeated falls.31-33 Conversely, there appears to be a physiological decrease in muscle strength with age, which is not prevented by supplementation with vitamin D3 4,35; it has been shown that multiple comorbidities also cause muscle weakness, which is improved by vitamin D supplementation.36
In a review of the causal mechanisms involved in falls, the authors observed an association of vitamin D deficiency with muscle disorders. However, when adjusted for other variables, such as age, physical activity, body mass index, and chronic diseases, this association disappeared.37 Neuromuscular improvement after supplementation, when it occurs, appears to depend mainly on levels prior to replacement.
Other studies demonstrated that: a) supplementation with vitamin D and calcium in 148 elderly women who were vitamin D deficient (< 20 ng/mL) resulted in a 9% improvement in stability and fewer falls during a one-year follow up, compared to calcium supplementation alone38; b) supplementation of 400 IU/day of vitamin D did not reduce falls in 354 Dutch institutionalized elderly individual aged > 70 years39; c) vitamin D supplementation (compared to placebo) did not influence falls in 389 elderly outpatients, although this study did not measure levels of vitamin D40; d) in a controlled study with 3,717 institutionalized elderly individuals, vitamin D supplementation did not reduce falls or fractures.41
The results of some of these studies have hypothesized that the lack of effect of vitamin D on falls in the elderly is due to the fact that they are institutionalized and therefore under better environmental control. However, Venning et al. stated that lack of improvement regarding falls with vitamin D supplementation, observed by some researchers, would not be due to this fact but to instead due to the low doses of vitamin D used in the supplementation.42 Indeed, studies with institutionalized elderly have shown improvement with doses of 800 IU/day, which does not occur when the replacement dose is 400 IU/day as discussed below. There is, however, no direct comparison between doses.
In a double-blinded, randomized study with 139 outpatients aged > 65 years who had a history of falls and vitamin D levels < 12 ng/mL, the study group received a single dose of 600,000 IU of intramuscular ergocalciferol. The time of functional neuromuscular performance, time of psychomotor reaction, and balance improved significantly. However, muscle strength did not change, and there was no difference in the frequency of falls. This only suggests a vitamin D action on neuromuscular function in patients with vitamin D deficiency, with no effect on falls.43
Finally, in a recent controlled study of annual supplementation with high doses of vitamin D (500,000 IU/year), Sanders et al. observed no reduction in falls among 2,256 non-institutionalized Australian elderly women. In fact, the falls even increased. In this study, basal levels of vitamin D were not different between the two groups44 (21 vs. 18 ng /mL).
Is this evidence conclusive in itself?
No. Methodological variations may be responsible for conflicting results, as the magnitude of the supplementation benefit depends on gender (women more than men); on whether the individual is institutionalized or not (non-institutionalized more than institutionalized); levels prior to supplementation (higher gain if basal levels are lower); dose (the benefit is greater with doses > 800 IU/day); and presence of multiple comorbidities (when there are no comorbidities, the benefit is greater).
If there is an association of vitamin D with falls, this occurs mainly in non-institutionalized elderly individuals with vitamin deficiency. This might be due to the fact that institutionalized women have multiple comorbidities that result in falls. The association of vitamin D deficiency with falls appears to be more due to the action of vitamin D on neuromuscular function than on muscle strength itself.
Are there systematic reviews of RCTs that evaluated vitamin D supplementation on the incidence and mortality of falls?
Yes. In a first meta-analysis, Bischoff-Ferrari et al. analyzed five randomized and controlled trials involving 1,237 elderly patients with stable health status, and concluded that supplementation with vitamin D reduces falls by 22% (OR = 0.78, 95% CI = 0.64 to 0.92) when compared to calcium supplementation or placebo, with a number needed to treat (NNT) of 15 during the study period. The effect was significant only in women. However, serum levels of vitamin D were not measured and the level of physical activity was not quantified; moreover, the vitamin D dose was not established, as well as the type and time of supplementation, or whether there was need for calcium association.45
In another meta-analysis of randomized trials, the reduction in falls was observed only when vitamin D was supplemented at a dose of 700 IU/day or more. In this analysis, the benefits occurred both in institutionalized patients and outpatients. The results showed that when serum levels were < 24 ng/mL, falls were not prevented. The concomitant supplementation with calcium did not influence the results. The difference between genders was not evaluated.46
Can it be concluded that low vitamin D levels are associated with higher incidence and morbimortality from falls, and that its replacement can prevent them?
Perhaps. In the case of mortality, the evidence is indirect, i.e., if there are fewer falls, as observed in some studies and meta-analyses, and the falls are associated with increased mortality, then there would be a reduction in mortality with vitamin D supplementation. Falls occur in 30% of those older than 65 years, resulting in approximately 5% of fracture cases, and in 40% to 50% of those older than 80 years in the United States. This is associated with high morbidity and mortality, as well as high costs.47,48
Discussion
The cause-effect association between lack of vitamin D and bone diseases such as childhood rickets and adult osteomalacia is already well-established. However, recently, the interest of the medical and scientific community turned to its possible association with extra-osseous clinical manifestations, whose consequences to public health can be significant.
The hypothesis that vitamin D deficiency is associated with the incidence or mortality caused by other diseases not primarily bone-related emerged in countries where prevention of skin cancer, with the use of sunscreen with a high protection factor, led to low levels of vitamin D in the population. This is what occurred in Australia.49
The sum of preliminary epidemiological findings and those from experimental studies gave rise to the concept of possible association between low serum vitamin D levels and several extra-osseous diseases. However, evidence of a cause-effect association between them still depends on scientific confirmation obtained from large studies with humans and the clarification of some unresolved issues.
The first of these issues is related to the reference values of normality, i.e., how these parameters were defined to classify the body load of vitamin D as normal, insufficient, or deficient, and how these categories correlate with different clinical manifestations that are supposedly related.
Firstly, the cutoff values for the serum levels of 25(OH) D3 were not established in relation to the incidence or prevalence of health problems of population groups. They were calculated from the simple correlation with serum PTH level, which is used as reference standard. In other words, levels of 25(OH)D3 < 20 ng/mL (which differentiates vitamin D insufficiency from deficiency, according to the most often used criteria) trigger an increase in PTH levels above that establi shed as normal (intermediate outcome), but they do not necessarily represent a higher risk of extra-osseous disease onset50 (final outcome).
At this point, the identification of the real "clinical significance" of the currently used reference values is crucial, especially when considering that population measurements of vitamin D performed in several countries have shown a high prevalence of low vitamin D levels, according to the normality, insufficiency, and deficiency criteria used to date. It is noteworthy that the main causes of high prevalence of low levels of vitamin D3 in the population are probably associated with habits and behaviors common to the modern lifestyle: sedentary life style, low exposure to sunlight, unbalanced diet, excessive accumulation of body fat, and abuse of topical or oral medications that interfere with vitamin D absorption or metabolism.
Thus, the preliminary findings triggered observational and intervention studies that have attempted to verify whether low vitamin D levels were associated with increased incidence of or mortality from extra-osseous diseases, and whether vitamin D supplementation could prevent them.
In general, a systematic review of observational studies (cohort or case-control studies, able to detect an association, but not causality) confirmed the possibility that hypovitami nosis D increases the incidence of or mortality from extra-osseous diseases, but in many cases, the results were inconsistent or even conflicting (Table 2).
Two questions that have not been answered adequately may be responsible for these findings: a) observational studies are subject to misinterpretation or incomplete interpretations if confounder variables are not adequately controlled. For instance, body fat accumulation, sedentary lifestyle, skin color, and nutritional status, can be, simultaneously, causes of vitamin D deficiency and risk factors for cancer, cardiovascular disease, diabetes, and low immunity, therefore casting doubts on the proposed associations; b) there is the possibility of a reverse bias: chronic diseases can lead to patient isolation, low sun exposure, physical inactivity, anorexia, and worsening of diet and nutritional status, which lead to decreased levels of circulating 25(OH)D3 . Thus, vitamin D measurement in these cases would serve more as a marker of active disease; and vitamin D deficiency, if diagnosed, would be the consequence, not the cause of the investigated disease.
The solution to such questions depends on the performance of RCTs, which are intervention studies in which the incidence of and/or mortality from the disease (outcomes) throughout the study duration are compared to randomly distributed groups of individuals in whom basal levels of 25(OH)D3 are measured, and dietary supplementation of vitamin D is evaluated in relation to a placebo (control group).
Table 2 shows the main results of RCTs and systematic reviews of the literature available to date. Contrary to the first impressions, there is no concrete, high methodological quality evidence to support the existence of a cause-effect between low levels of vitamin D and cancer, cardiovascular disease, and diabetes mellitus type 2, just as there is no conclusive evidence that vitamin D supplementation is able to prevent them. Some partial results of larger studies, however, still maintain these possibilities open to investigation, which deserve to be further studied.
Although the data shown in this study has demonstrated the lack of evidence for a safe recommendation for vitamin D research and/or supplementation, some international societies have made specific recommendations. The United States Preventive Services Task Force (USPSTF) recommends vitamin D supplementation for all individuals older than 65 at risk for falls, regardless of the dose51 (Recommendation B). The recommended doses vary according to the Society. The Institute of Medicine recommends 600 IU for individuals aged 51 to 70 years and 800 IU for those older than 70 years.52 The American Geriatrics Society recommends 800 IU for all individuals at risk of falling.53 The American Endocrine Society recommends the measurement of vitamin D3 in certain clinical situations including chronic use of medications (anticonvulsants, corticosteroids, antiretrovirals, and antifungal drugs), in pregnant women and infants, Blacks, Hispanics, obese individuals (regardless of age), and elderly individuals with a history of falls and non-traumatic fractures, osteoporosis, mal-absorption, and granulomatous diseases. It also recommends the measurement in patients with rickets, chronic kidney disease, and liver disease[8] - that is, to a significant portion of the population.
It is worth emphasizing that the results of the available studies are scarce and incomplete. Among the more obvious limitations, the following should be noted: a) not all diseases among those analyzed in the present study were assessed as the primary outcome in the available studies and reviews; b) the study populations were heterogeneous, and the results cannot always be extrapolated to the general population; c) the basal levels of 25(OH)D3 were not always measured, and the dose and type of vitamin D supplementation varied significantly among the studies; d) the presence of comorbidities and consumption of chemical substances among the participants were not always adequately controlled; and e) time of follow-up in most studies may have been insufficient. As there are hundreds of ongoing registered RCTs on the subject around the world, within a few years valuable information should be available to guide clinical practice.
For the time being, healthy lifestyle habits, such as regular physical activity, moderate sun exposure, balanced diet, weight control, and controlled consumption of chemical substances still appear to be the most effective way to promote health and prevent disease, whether or not related to hypovitaminosis D.
Conclusion
Based on the best scientific evidence available to date, it is not possible to establish a causal association between low serum levels of 25(OH)D3 and cancer, cardiovascular disease, and diabetes mellitus type 2. Therefore, vitamin D routine measurement or dietary supplementation is not recommended for preventive purposes in the general population. It is possible that periodic measurement of 25(OH)D3 and vitamin supplementation, when necessary, can bring benefits to women older than 60 to 65 years, non-institutionalized, with a history or high risk of repeated falls.
Financial support
Fundação Faculdade de Medicina da Universidade de São Paulo.
Conflicts of interest
All authors declare to have no conflicts of interest.
REFERENCES
- 1. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50-60.
- 2. Holick MF. Vitamin D Deficiency. N Engl J Med. 2007;357:266-81.
- 3. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-8.
- 4. Kimball S, Fuleihan GEH, Vieth R. Vitamin D: a growing perspective. Crit Rev Clin Lab Sci. 2008;45:339-415.
- 5. Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: A comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. 2012;58:531-42.
- 6. Schuch NJ, Garcia VC, Martini LA. Vitamina D e doenças endocrinometabólicas. Arq Bras Endocrinol Metab. 2009;53:625-33.
- 7. Adams JS, Hewison M. Update in Vitamin D. J Clin Endocrinol Metab. 2010;95:471-8.
- 8. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon GM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of Vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911-30.
- 9. Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer: ready for prime time? N Engl J Med. 2011;364:1385-7.
- 10. Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with and without calcium supplementtion for prevention of cancer and fractures: un updated meta-analysis for the US Preventive Services Task Force. Ann Intern Med. 2011;155:827-38.
- 11. Helzlsouer KJ. Overview of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:4-9.
- 12. Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, O'Sullivan MJ, Margolis KL, et al., for the Women's Health Initiative Investigators. Calcium plus Vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684-96.
- 13. Park S, Lee BK. Vitamin D deficiency is an independent risk factor for CVD in koreans aged > = 50 years: results from the Korean National Health and Nutrition Survey. Nutr Res Pract. 2012;6:162-8.
- 14. Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-14.
- 15. Jorde R, Schirmer H, Wilsgaard T, Joakimsen RM, Mathiesen EB, Njolstad I, Lochen ML, et al. Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality. The Tromsø Study. PLoS One. 2012;7:e37295.
- 16. Rejnmark L, Avenell A, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70528 patients from major vitamin D trials. J Clin Endocrinol Metab. 2012;97:2670-81.
- 17. Gepner AD, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of Vitamin D supplementation on cardiovascular disease risk. PLoS One. 2012;7:e36617.
- 18. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. Review: the role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017-29.
- 19. Ishii H, Suzuki H, Baba T, Nakamura K, Watanabe T. Seasonal variation of glycemic control in type 2 diabetic patients. Diabetes Care. 2001;24:1503.
- 20. Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771-7.
- 21. Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, et al. Levels of Vitamin D in cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65:225-36.
- 22. De Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, for the Women's Health Initiative Investigators. Calcium plus Vitamin D supplementation and the risk of incident diabetes in the women's health initiative. Diabetes Care. 2008;31:701-7.
- 23. Hurst PR, Stonehouse W, Coad J. Vitamin D reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient- a randomized, placebo-controlled trial. Br J Nutr. 2010;103:549-55.
- 24. Janssen HCJP, Samson MM, Verhaar HJJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611-5.
- 25. Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882-91.
- 26. Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stähelin HB, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19-24.
- 27. Sorensen OH, Lund B, Saltin B, Lund B, Andersen RB, Hjorth L, et al. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond). 1979;56:157-61.
- 28. Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev. 1986;7:434-47.
- 29. Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113-8.
- 30. Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343-51.
- 31. Stein MS, Wark JD, Scherer SC, Walton SL, Chick P, Di Carlantonio M, et al. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J Am Geriatr Soc. 1999;47:1195-201.
- 32. Gloth MF, Tobin JD. Vitamin D deficiency in older people. J Am Geriatr Soc. 1995;43:822-8.
- 33. Thomas MK, Lloyd-Jones MD, Thadhadi RI, Shaw AC, Deraska JD, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777-83.
- 34. Grady D, Halloran B, Cummings S, Leveille S, Wells L, Black D, et al. 1,25-Dihydroxyvitamin D3 and muscle strength in the elderly: a randomized controlled trial. J Clin Endocrinol Metab. 1991;73:1111-7.
- 35. Johnson KR, Jobber J, Stonawski BJ. Prophylactic vitamin D in the elderly. Age Ageing. 1980;9:121-7.
- 36. Corless D, Dawson E, Fraser F, Ellis M, Evans SJ, Perry JD, et al. Do vitamin D supplements improve the physical capabilities of elderly hospital patients? Age Ageing. 1985;14:76-84.
- 37. Annweiler C, Montero-Odasso M, Schott AM, Berrut G, Fantino B, Beauchet O. Fall prevention and vitamin D in the elderly: an overview of the key role of the non-bone effects. J Neuroeng Rehabil. 2010;7:50-62.
- 38. Graafmans WC, Ooms ME, Hofstee HMA, Bezemer PD, BouterLM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143:129-36.
- 39. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670-6.
- 40. Law M, Winthers H, Morris J, Anderson F. Vitamin D supplementation and the prevention of fractures and falls: results of a randomized trial in elderly people in residential accommodation. Age Ageing. 2006;35:482-6.
- 41. Venning G. Recent developments in vitamin D deficiency and muscle weakness among elderly people. BMJ. 2005;330:524-6.
- 42. DhesiJK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589-95.
- 43. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral Vitamin D and falls and fractures in older women: a Randomized Controlled Trial. JAMA. 2010;303:1815-22.
- 44. Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of Vitamin D on falls a meta-analysis. JAMA. 2004;291:1999-2006.
- 45. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692.
- 46. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701-7.
- 47. Campbell AJ, Reinken J, Allan BC, Martinez GS. Falls in old age: a study of frequency and related clinical factors. Age Ageing. 1981;10:264-70.
- 48. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87 Suppl:1080S-6S.
- 49. Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of Vitamin D insufficiency in an adult normal population. Osteoporosis Int. 1997;7:439-43.
- 50. Prevention of falls in community-dwelling older adults: U.S. Preventive Services Task Force recommendation statement. 2012. Available from: http://tinyurl.com/6ovdmzc [acessado em 3 Dez 2012]
- 51. Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Institute of Medicine; 2011. Available from: http://www.nap.edu/catalog.php?record_id=13050 [acessado em 11 Dez 2012]
- 52. American Geriatrics Society, British Geriatrics Society. 2010 AGS/BGS Clinical Practice Guideline: prevention of falls in older persons. New York (NY): American Geriatrics Society; 2010. Available from: http://www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/2010/http://tinyurl.com/6ovdmzc[acessado em 14 Dez 2012]
Vitamin D: non-skeletal actions and rational use
Publication Dates
-
Publication in this collection
28 Nov 2013 -
Date of issue
Oct 2013
History
-
Received
02 Jan 2013 -
Accepted
03 May 2013