Abstract
A new species of Salicaceae growing in the state of Espírito Santo and in the south of the state of Bahia, Casearia valenciana R.Marquete & R.B.Torres, is described and illustrated here. It is similar to C. cotticensis Uittien, which grows further north in Amazonian Forest, by its pedunculate inflorescence, coriaceous leaves, and glabrous ovary. Yet, it differs from C. cotticensis by its shrub to small tree habit (vs. tree up to 27 m tall), smaller stipules, elliptic leaves (vs. lanceolate, broadly lanceolate, oblong-lanceolate), elongated anthers with longitudinal slits (vs. slightly deltoid, transversal slits), and larger fruits and seeds. Other diagnostic features of this new species include short-serrulate leaf border, and disc lobes fused with filaments at the base forming a small tube.
Key words Casearia valenciana; Salicaceae; Flacourtiaceae s.l.; restinga
Resumo
Casearia valenciana R.Marquete & R.B.Torres é uma nova espécie de Salicaceae que ocorre no Espírito Santo e sul da Bahia, aqui descrita e ilustrada. É semelhante a C. cotticensis Uittien, que possui distribuição mais ao norte, na Floresta Amazônica, pela inflorescência pedunculada, folhas coriáceas e ovário glabro. Difere daquela espécie pelo hábito arbustivo a pequena árvore (vs. árvore até 27m alt.), estípulas menores, folhas elípticas (vs. lanceolada, largo-lanceolada, oblongo-lanceolada), anteras alongadas com rimas longitudinais (vs. levemente deltoides, rimas transversais) e frutos e sementes maiores. Outros caracteres diagnósticos desta nova espécie são os bordos das folhas curto serrulado e lobos do disco e filetes fundidos na base, formando um pequeno tubo.
Palavras-chave Casearia valenciana; Salicaceae; Flacourtiaceae s.l.; restinga
Introduction
Casearia Jacq. is a genus with pantropical distribution and approximately 180 species in Africa and South and Central America. It grows in different types of vegetation, including dense ombrophilous forest, semi-deciduous forest, mixed ombrophilous forest, savanna, and steppe savanna. According to Castillo-Campos & Abreo (2003), 75 species occur in Southern and Central America. These data agree with those from Flora Neotropica (Sleumer 1980), according to which 45 species grow in Brazil. Marquete & Mansano (2010, 2013) described two new species in the genus and, later, when reviewing its Brazilian species they accepted a total of 48 species (Marquete & Mansano 2016). Casearia is characterized by tree, shrub or subshrub, branches patent or erect, rarely thorny; stipule usually caducous; leaves alternate, distichous, generally with pellucid dots and or lines; inflorescence fascicle, glomerule, rarely cyme; flowers hermaphrodite, sepals (4–)5(–9), free or united at base, stamens (6–)8–10(rarely –25), uniseriate, anthers globose to ovoid, disk lobes inter, intra or extra the row of stamens; ovary free, 1-locular, style entire or triphid at apex, stigma simple or trilobate; capsule dry or succulent, embryo straight. Increased fieldwork in Brazil revealed new taxa that were described and published, particularly from the Atlantic Forest (Marquete & Mansano 2010, 2013; Nepomuceno & Alves 2017). Here we describe and illustrate a new species that can be recognized by its glabrous leaves on both faces, short-serrulate leaf border, stipules with scattered trichomes on the margins and surface, elongated glands on the surface, larger peduncle (4–4.5 mm long), lobes of the disc forming a small tube, and anthers oblong with a longitudinal slit.
Material and Methods
The description of the species was based on material from the following herbaria: CEPEC, CVRD, HRB, IAC, INPA, MBML, RB, VIES (acronyms according to Thiers (continuously updated). Specimens were analyzed using a Zeiss Stemi SV11 stereoscopic microscope. The measurements were based on rehydrated material. The terminology adopted in the description followed Font Quer (1979) and Hickey et al. (2000). A morphological comparison of Casearia valenciana and C. cotticensis is presented. In the protologue the holotype number refers to the number collection of INPA herbarium, and the isotype to the HRB barcode. The conservation status of the species was defined according to IUCN criteria (2019), and the GeoCAT (Bachman et al. 2011) tools. GeoCAT was applied with the IUCN default values for Extent of Occurrence (EOO) and Area of Occupancy (AOO) analysis. When the herbarium specimens were not geo-referenced, the geographic coordinates were those of the count (CRIA 2021).
Distinguishing characters between Casearia valenciana R.Marquete & R.B.Torres and its morphologically similar species, C. cotticensis Uittien.
Results and Discussion
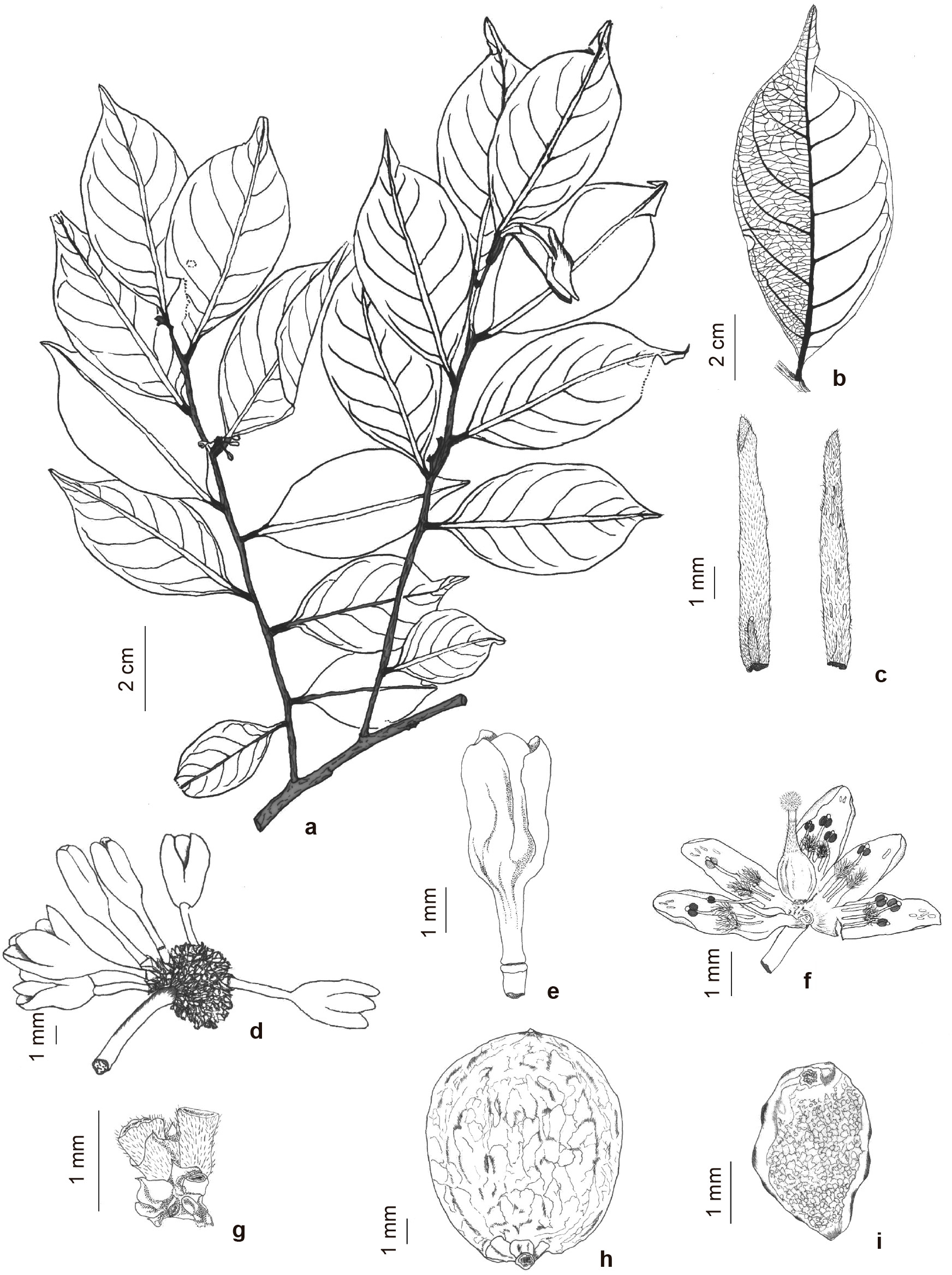
Casearia valenciana R.Marquete & R.B.Torres, sp. nov. Type: BRAZIL. BAHIA: Valença, km 9 da estrada Valença–Guaibim, 06.II.1983, André M. de Carvalho & T. Plowman 1483 (holotypo: INPA 111674!; isotypo: HRB 11361!). Fig. 1
a-i. Casearia valenciana – a. branches with inflorescence in buds; b. detail of dense reticulate leaf venation; c. stipules with elongated glands, inner and outer surfaces; d. inflorescence showing peduncle, pedicels, flower, and flower buds; e. flower bud with articulated pedicel; f. open flower with filaments alternating with disc lobes, anthers with an apical gland, ovary with a gland on its surface, and hirsute stigma; g. bracts and bracteoles with apical glands; h. fruit; i. seed with a laciniate aril. Drawn by R. Marquete, based on A.M. de Carvalho 1483.
The new specie Casearia valenciana differs from C. cotticensis Uittien in its habit (shrub to tree (up to 12 m vs. tree up to 27 m high), by the glabrous branches (vs. sparse villous near the apex, glabrescent near the base), leaf (elliptic, apex wide mucronate to cuspidate apiculate, margin short serrulate vs. lanceolate, broadly lanceolate or oblong lanceolate, apex caudate to short caudate, margin serrate to serrulate), stipules with trichomes on the edges (vs. tomentose), larger peduncle (4–4.5 mm vs. 2–3 mm long), lobes of the disc forming a small tube, and by the oblong anthers with a longitudinal slit (vs. slightly deltoid, transversal slit).
Shrub 2–4 m to tree 7–12 m tall; trunk unarmed, bark smooth. Branches cylindrical, glabrous, grayish, lenticels globose to elongated, scattered on branches; stipules (4–) 8–9 (–10) × 1 mm, linear-lanceolate, indument scattered on edges and surface, chartaceous, elongated glands on inner face at the base, border and the apex, scattered on outer surface, caducous. Leaves persistent, discolored, opaque on both faces, shape and size with little variation along the branch; blade 9–10.5 × 4–4.5 cm, elliptic, apex wide-mucronate to cuspidate apiculate, base attenuate, margins short-serrulate, glabrous, coriaceous, visible lines and punctuations translucent near the margins, secondary veins 7–9 (–12) pairs ascending, slightly prominent adaxially, prominent abaxially, dense-reticulate; petioles 3–4 (–8) mm, subcylindrical, glabrous, blackish. Inflorescences umbelliform, + 40 flowers per axil, peduncle (3–) 4–4.5 mm long, glabrous, greenish; bracts and bracteoles 0.5–0.7 (–1) mm long, involving the pedicel base, ovate, indument sparsely hirtellous, dark yellow; pedicels 2.5–3 (–4) mm long, cylindrical, hirtellous, dark yellow, articulate 1 mm from the base. Flower buds 2–3 × 1–2 mm, oblong, adpressed-pubescent to glabrescent. Sepals 5, 3–4 × 1.5 mm, oblong to ovate, adpressed-pubescent on both faces, white, with elongated glands; stamens 10, alternately short and long, glabrous, filaments 0.8–1 mm, subcylindrical to flattened at base, fused to disc lobes and sepals at the base, thecae oblong, glabrous, separated by an apical and rounded gland, longitudinal slits; disc lobes approximately 0.8 mm, clavate, flattened, tomentous at the apex to glabrescent at the base, intercalated and fused with filaments at the base, forming a small tube (1 mm long); ovary ovate, glabrous, style entire, short, pubescent and thick on insertion of the ovary and glabrescent towards the apex, stigma capitate, entire, hirsute. Capsules 6,5–5-9.2 × 6.0–8.0 mm, oblong-elliptical, glabrous, green when immature, indument persistent at style insertion; seeds 2.5–3 × 1.1–2.15 mm, oblong to angular, orange to yellow; aril thin, orange-yellow; seed testa foveolate, orange; embryo straight with rounded cotyledons leaves, with cylindrical radicle hypocotyl axis.
Examined material: BRAZIL. Bahia: Porto Seguro, Reserva Florestal de Porto Seguro – CVRD, 3.VII.1990, bt & fl., D.A. Folli 1159 (CVRD). Valença, ramal à esquerda da rodovia que liga Valença ao Guaibim (litoral), com entrada no km 9, 11.XII.1980, fr., L.A. Mattos Silva et al. 1265 (RB, CEPEC); 6.II.1983, fl., A.M. de Carvalho & T. Plowman 1483 (INPA, CEPEC). Espírito Santo: Divino de São Lourenço, mata fria, terreno de Clério Loss, 28.X.1998, fl., L. Kollmann et al. 812 (IAC, MBML); 28.X.1998, fl, L. Kollmann et al. 843 (MBML, RB). Linhares, Reserva Biológica de Comboios, formação de mata seca, 19.6719o S x 39.8828o W, 14.V.1995, fl., I. Weiler Júnior 299 (VIES); estrada ligando Pontal do Ipiranga a Degredo, 5.III.2008, fr., O.J. Pereira et al. 7553 (VIES). Santa Maria de Jetibá, Rio Nove, terreno de L. Kollmann, 20.I.1999, fr., L. Kollmann & Bausen 1612 (IAC, MBML). Santa Teresa, Aparecidinha, terreno de Luis Brigenti, 6.X.1998, bot., L. Kollmann et al. 696 (IAC, MBML); Estação Biológica de Santa Lucia, 22.IX.1993, bot. &fl., L.D. Thomaz 1786 (IAC, MBML); Reserva da Prefeitura, Estrada Caravage (Caravaggio), 27.X.1998, fl., L. Kollmann et al. 800 (IAC, MBML); Estação Biológica de São Lourenço (da caixa d’água), Estrada do Caravage, 18.XI.1998, fl., L. Kollmann et al. 1050 (IAC, MBML). Vitória, Santo Antônio, terreno do Boza, 17.XI.1998, fl., L. Kollmann et al. 1033 (IAC, MBML); 7.I.1999, fr., L. Kollmann & E. Bausen 1511 (IAC, MBML).
Casearia valenciana species occurs in the states of Bahia and Espírito Santo, Brazil. It grows in restinga, semideciduous seasonal Atlantic Forest, forest edge on rocky outcrop, and lowland dense ombrophylous forests, at 10 meters altitude.
Casearia valenciana has an AOO of 28 km2 (EN) and an EOO of 74.974 km2 (LC). Although the species occurs in protected areas, it can be classified as vulnerable (VU – B2aiii) since it occurs in less than 10 localities. Furthermore, C. valenciana occurs on highly fragmented landscapes (Rezende et al. 2018) and is poorly represented in herbaria.
In Brazil, the Casearia cotticensis is restricted to the region of dense, ombrophilous Amazon Forest, while C. valenciana was found in restinga and in lowland dense ombrophilous forests.
Casearia valenciana occurs in an area of high endemism of the Atlantic Forest, the biogeographic region of Bahia (Silva & Casteleti 2005), where taxa of Amazonian origin are recorded (Peixoto & Gentry 1990). Different authors (Costa 2003; Silva & Casteleti 2005; Fiaschi & Pirani 2009; Sobral-Souza et al. 2015) consider that until the Tertiary period, when the decrease of humidity and the increase of climate aridity occurred, the Atlantic Forest and the Amazon Rainforest probably formed a continuum. Thus, we hypothesize that C. valenciana and C. cotticensis could form a pair of vicariant species.
The species is collected with flowers from February to May and with fruits in March and, tardily, in December.
According to Sleumer (1980), Casearia valenciana belongs to sect. Casearia, group Arboreae. The groups suggested by the author have no nomenclatural status but they facilitate the identification of the numerous and similar species of Casearia.
The specific epithet is a homage to the city where the plant was collected for the first time.
Acknowledgements
This paper was prepared at the Instituto de Pesquisas Jardim Botânico do Rio de Janeiro and Instituto Agronômico de Campinas, Brazil. The authors are grateful to the curators of the mentioned herbaria, to Alan P.A. François for helping with the English version, and to Patrícia da Rosa for helping with GeoCAT.
References
- Bachman S, Moat J, Hill AW, Torre J & Scott B (2011) Supporting Red List threat assessments with GeoCAT: geospatial conservation assessment tool. In: Smith V, Penev L (eds.) e-Infrastructures for data publishing in biodiversity science. ZooKeys 150: 117-126. Avaliable at http://geocat.kew.org/. Access on 10 August 2021.
- Castillo-Campos G & Abreo MEM (2003) A new species of Casearia (Flacourtiaceae) from Mexico. Novon 13: 30-33.
- Costa LP (2003) The historical bridge between the Amazon and the Atlantic Forest of Brazil: a study of molecular phylogeography with small mammals. Journal of Biogeography 30: 71-86.
-
CRIA - Centro de Referência e Informação Ambiental 2021. Specieslink - simple search. Avaliable at http://www.splink.org.br/ Access on 07 August 2021.
» http://www.splink.org.br/ - Fiaschi P & Pirani JR (2009) Review of plant biogeographic studies in Brazil. Journal of Systematics and Evolution 47: 477-496.
- Font Quer (1979) Diccionario de botánica. Editorial Labor, Barcelona. 1280p.
- Hickey M, King C & M King (2000) The Cambridge illustrated glossary of botanical terms. Cambridge University Press, Cambridge. 208p.
-
IUCN (2019) IUCN. Standards and petitions committee. Guidelines for using the IUCN Red List categories and criteria. V. 14. Prepared by the Standards and Petitions Committee. Avaliable at <http://www.iucnredlist.org/documents/RedListGuidelines.pdf>.Access on 06 August 2021.
» http://www.iucnredlist.org/documents/RedListGuidelines.pdf - Marquete R & Mansano VF (2010) A new species of Casearia (Salicaceae) from Southeastern Brazil. Novon 20: 179-181.
- Marquete R & Mansano VF (2013) A new species of Casearia (Salicaceae) from Brazil. Journal of Systematics and Evolution 51: 228-229.
- Marquete R & Mansano VF (2016) O gênero Casearia Jacq. no Brasil. Revista de Biologia Neotropical 13: 69-249.
- Nepomuceno FAA & Alves M (2017) A new Casearia (Salicaceae) from the Atlantic Forest of Brazil. Phytotaxa 311: 297-300.
- Peixoto AL & Gentry A (1990) Diversidade e composição florística da mata de tabuleiro na Reserva Florestal de Linhares (Espírito Santo, Brasil). Revista Brasileira de Botânica 13: 19-25.
- Rezende CL, Scarano FR, Assad ED, Joly CA, Metzger JP, Strassburg BNN, Tabarelli M, Fonseca GA & Mittermeier RI (2018) From hotspot to hopespot: an opportunity for the Brazilian Atlantic Forest. Perspectives in Ecology and Conservervation 16: 208-214.
- Silva JMC & Casteleti CHM (2005) Estado da biodiversidade da Mata Atlântica brasileira. In: Galindo-Leal C & Câmara IG (eds) Mata Atlântica biodiversidade, ameaças e perspectivas. Fundação SOS Mata Atlântica, Conservação Internacional e Centro de Ciências Aplicadas à Biodiversidade, Belo Horizonte. Pp. 43-59.
- Sleumer HO (1980) Flacourtiaceae. Flora Neotropica. Monograph 22: 1-499.
- Sobral-Souza T, Lima-Ribeiro M & Solferini VN (2015) Biogeography of Neotropical Rainforests: past connections between Amazon and Atlantic Forest detected by ecological niche modeling. Evolutionary Ecology 29: 643-655.
-
Thiers BM (continuously updated) Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Available at <http://sweetgum.nybg.org/science/ih/>. Access on 10 January 2021.
» http://sweetgum.nybg.org/science/ih/
Edited by
-
Area Editor: Dr. Rafael Pinto
Publication Dates
-
Publication in this collection
16 May 2022 -
Date of issue
2022
History
-
Received
7 Apr 2021 -
Accepted
01 Sept 2021

 A new species of Casearia Jacq. from Brazil
A new species of Casearia Jacq. from Brazil