Abstracts
Anthocyanins show low-stability when exposed to different food processing conditions. Copigmentation is one of the main reactions contributing to the in vivo color responsible to the stability of anthocyanins. In the aim of holding the red color, copigmentation effect of organic acids (caffeic, ferulic, gallic and tannic acids) combined with anthocyanins in crude Cabernet Sauvignon (Vitis vinifera L.) grape skin extract at pH values (1.0, 2.0, 3.0, 3.3, 3.5, 3.7, 4.0, 4.5) was evaluated in this research. The maximum copigmentation effect, revealed by the hyperchromic and bathochromic shifts in anthocyanin maximum absorbance wavelength, was obtained at pH 3.3 with every acid used. Anthocyanin stability was followed by measuring the loss of color, thus it was possible to determine the protecting effects of these copigments. Tannic acid was the best copigment in our model system, giving half-life time of 2,585 h. We are suggesting the formation of pyranoanthocyanins by the reactions of anthocyanins with caffeic and ferulic acid, these substances could be avoiding the observation of the copigmentation effect. Addition of organic acids could improve the anthocyanin stability; though, more studies are needed to justify the lack of copigmenting effect observed with the caffeic and ferulic acids.
stability; pigment; color retention; half-life time
Antocianinas apresentam baixa estabilidade frente aos fatores que afetam o processamento de alimentos. A copigmentação é uma das principais reações responsáveis pela estabilidade da coloração de antocianinas in vivo. Com objetivo de manter a coloração vermelha, a copigmentação das antocianinas do extrato bruto de uvas Cabernet Sauvignon (Vitis vinifera L.) com ácidos orgânicos (ácidos caféico, ferrúlico, gálico e tânico) em diferentes valores de pH (1,0; 2,0; 3,0; 3,3; 3,5; 3,7; 4,0; 4,5) foi avaliada neste estudo. O efeito máximo de copigmentação, revelado pelos deslocamentos hipercrômico e batocrômico, foi obtido em pH 3,3 para todos os ácidos orgânicos utilizados. A estabilidade da cor das antocianinas foi avaliada, sendo possível observar o efeito protetor da cor das antocianinas pelos copigmentos, que foi mais efetivo quando o ácido tânico foi adicionado às soluções, conferindo tempo de meia vida de 2.585 h. A possível formação de piranoantocianinas a partir da reação das antocianinas com os ácidos caféico e ferrúlico pode ter mascarado o efeito protetor da cor pela reação de copigmentação. A adição de ácidos orgânicos é uma ferramenta para a estabilização de antocianinas; no entanto mais estudos são necessários quanto à formação das piranoantocianinas, que diminuem a intensidade da cor vermelha das soluções.
estabilidade; pigmento; retenção de cor; tempo de meia vida
FOOD SCIENCE AN TECHNOLOGY
Effect of pH on the copigmentation of anthocyanins from Cabernet Sauvignon grape extracts with organic acids
Efeito do pH na copigmentação de antocianinas do extrato de uvas Cabernet Sauvignon com ácidos orgânicos
Cony Gauche; Elisa da Silva Malagoli; Marilde Terezinha Bordignon Luiz * * Corresponding author < bordign@cca.ufsc.br>
UFSC - Depto. de Ciência e Tecnologia de Alimentos, Rod. Admar Gonzaga 1346 - 88034-001 - Florianópolis, SC - Brasil
ABSTRACT
Anthocyanins show low-stability when exposed to different food processing conditions. Copigmentation is one of the main reactions contributing to the in vivo color responsible to the stability of anthocyanins. In the aim of holding the red color, copigmentation effect of organic acids (caffeic, ferulic, gallic and tannic acids) combined with anthocyanins in crude Cabernet Sauvignon (Vitis vinifera L.) grape skin extract at pH values (1.0, 2.0, 3.0, 3.3, 3.5, 3.7, 4.0, 4.5) was evaluated in this research. The maximum copigmentation effect, revealed by the hyperchromic and bathochromic shifts in anthocyanin maximum absorbance wavelength, was obtained at pH 3.3 with every acid used. Anthocyanin stability was followed by measuring the loss of color, thus it was possible to determine the protecting effects of these copigments. Tannic acid was the best copigment in our model system, giving half-life time of 2,585 h. We are suggesting the formation of pyranoanthocyanins by the reactions of anthocyanins with caffeic and ferulic acid, these substances could be avoiding the observation of the copigmentation effect. Addition of organic acids could improve the anthocyanin stability; though, more studies are needed to justify the lack of copigmenting effect observed with the caffeic and ferulic acids.
Key words: stability, pigment, color retention, half-life time.
RESUMO
Antocianinas apresentam baixa estabilidade frente aos fatores que afetam o processamento de alimentos. A copigmentação é uma das principais reações responsáveis pela estabilidade da coloração de antocianinas in vivo. Com objetivo de manter a coloração vermelha, a copigmentação das antocianinas do extrato bruto de uvas Cabernet Sauvignon (Vitis vinifera L.) com ácidos orgânicos (ácidos caféico, ferrúlico, gálico e tânico) em diferentes valores de pH (1,0; 2,0; 3,0; 3,3; 3,5; 3,7; 4,0; 4,5) foi avaliada neste estudo. O efeito máximo de copigmentação, revelado pelos deslocamentos hipercrômico e batocrômico, foi obtido em pH 3,3 para todos os ácidos orgânicos utilizados. A estabilidade da cor das antocianinas foi avaliada, sendo possível observar o efeito protetor da cor das antocianinas pelos copigmentos, que foi mais efetivo quando o ácido tânico foi adicionado às soluções, conferindo tempo de meia vida de 2.585 h. A possível formação de piranoantocianinas a partir da reação das antocianinas com os ácidos caféico e ferrúlico pode ter mascarado o efeito protetor da cor pela reação de copigmentação. A adição de ácidos orgânicos é uma ferramenta para a estabilização de antocianinas; no entanto mais estudos são necessários quanto à formação das piranoantocianinas, que diminuem a intensidade da cor vermelha das soluções.
Palavras-chave: estabilidade, pigmento, retenção de cor, tempo de meia vida.
Introduction
Anthocyanins play a critical role in the color quality of many fresh and processed fruits. The reasons for increasing the use of these colorants could be justified by their beneficial health effects (Torskangerpoll and Andersen, 2005). Anthocyanins have a useful potential as natural colorants due to their attractive colors (Markakis, 1982); however, their usage in the food industry is limited due to their instability when exposed to factors like environmental variations, including temperature, light intensity and oxygen (Delgado-Vargas et al., 2000). A particular problem is the pH influence on their behavior (Fossen et al., 1998). Anthocyanins differ from other natural flavonoids due to their large range of colors and their ability to form resonance structures through pH variation (Lapidot et al., 1999). Some extracted anthocyanins have been used as colorings for food and beverages; however, many anthocyanins are unstable in neutral solutions and lose their color, thus their use for food and pharmaceutical products, among others, has been limited (Dougall and Baker, 2008).
Copigmentation represents an important factor of anthocyanin chromophore stabilization in in vivo systems (Baranac et al., 1997). In plant vacuoles, where pH is close to 7.0, the intense observed blue/red colorations have been attributed to complexation of the anthocyanins with other flavonoids, phenolics and/or metal ions, like Al3+ or Mg2+ (Dangles et al., 1994). The exact mechanisms of copigmentation are poorly known. Some copigments may protect the C-2 chromophore in the pyranic ring against water's nucleophilic attack, which often leads to color loss (Awika, 2008).
The objective of this research was to evaluate the influence of pH and organic acids on the stability of anthocyanins from crude extract of Cabernet Sauvignon (Vitis vinifera L.) grape skin.
Material and Methods
The crude extract of grape skin used for this research was obtained from red grapes (Vitis vinifera L.) from Cabernet Sauvignon cultivars from a vineyard located in São Joaquim (28°17' S, 49°55' W, 1,415 m a.s.l.), state of Santa Catarina, Brazil. Tannic acid and gallic acid were obtained from Vetec Ltda (Rio de Janeiro, Brazil); caffeic acid and ferulic acid were obtained from Fluka (³95% HPLC). All others reagents were of analytical degree.
Anthocyanin extraction and quantification
The grapes were washed in running water and then in water containing 200 mg kg-1 sodium hypochlorite. Grape skins were separated from the pulp, bleached in boiling water for 2 min and frozen at -18 ± 1°C in polyethylene bags. The crude extract of grape skin was extracted by the method described by Lees and Francis (1972); 100 g of grape skins were extracted, in darkness, with 400 mL of 0.1% HCl in methanol at 4.0 ± 1°C. The crude extract obtained was filtered through a nylon filter, and the skin remained was washed with 100 mL of the extracting solvent. A second vacuum filtration was carried out on Whatman (n. 2) filter paper in a Büchner funnel and afterwards the extract was concentrated under vacuum (Büchi Rotaevaporator-R-144) at 35°C until reaching the 50% of the methanol initial volume. The concentrated extract was filtered through a 0.45 µm Millipore filter and kept in an amber flask at -18.0 ± 1°C. The quantification of total anthocyanin content in the crude extract was measured by the pH-differential method (Giusti and Worsltad, 2001) using MW = 562.5 and e = 29,500 (Giusti et al., 1999) and expressed in g L1 of malvidin 3-glucoside, the main anthocyanin from Cabernet Sauvignon (Vitis vinifera L.) grapes (Passamonti et al., 2003; Wulf and Nagel, 1978).
Stability Study
Total solid content of the crude extract was estimated by drying 3 mL of anthocyanin crude extract at 105°C until reaching constant weight (AOAC, 2005). This result was utilized as an initial parameter to determine the amount of organic acid to be added in different weight/volume ratios (w v-1, organic acid/ crude extract anthocyanin). Control samples (model system without organic acid) and test samples (model system with organic acid) were prepared as it follows: the crude extract was placed in volumetric vials (25 mL) with 0.05% potassium sorbate (g L-1) to prevent a bacterial growth. The volume was completed with the model system of potassium chloride buffer (0.025 M chloride acid, potassium chloride) or citrate buffer (0.1 M citric acid, sodium citrate) at different pH values (1.0, 2.0, potassium chloride buffer; 2.5, 3.0, 3.3, 3.5, 3.7, 4.0, 4.5, citrate buffer) to obtain maximum absorbance values of 1,200 by the visible maximal wavelength displayed in the UV-visible spectra (Hitachi U2010, CA, USA). Solutions were then transferred to vials with lids (25 mL) after was shaken for 30 min and then rest for 2 h to reach equilibrium (Fuleki and Francis, 1968). The experiment was carried out in triplicate, with two replications. Test and control samples were kept under the following conditions: at a temperature of 28 ± 1°C (room temperature), at pH 1.0, 2.0, 2.5, 3.0, 3.3, 3.5, 3.7, 4.0, 4.5, under fluorescent lamps (2,500 lumens). The absorption spectra of the samples were monitored through UV-Vis absorption spectrophotometry in the visible wavelength range from 400 to 700 nm, at regular times, until 60% or more of the pigments were degraded.
Kinetic Study
For all samples, the half-life time (t1/2) values were calculated according to Kirca and Cemeroglu (2003), and the color retention percentage (%R) according to the following equations found in Katsaboxakis et al. (1998):
where t = time (hours); At = final absorbance ("time t"); A0 = initial absorbance ("time zero").
Statistical Analysis
The half-life time and color retention percentage of the samples under different parameters was studied by analysis of variance (ANOVA/MANOVA) with the STATISTICA Software version 6.0 (StatSoft, Inc. 1984-2001, Tulsa, OK74101 USA). Tukey Test was applied whenever a difference was detected among the factors (p < 0.05).
Results and Discussion
The total anthocyanin content in the crude extract was calculated as 1.33 g L-1, expressed as malvidin 3-glucoside equivalents. The total ratio of solids of the crude extract was estimated to be 23.8 g L-1. Anthocyanin concentration remained the same at every pH value studied (0.6 mL crude extract / 50 mL buffer).
The effect of pH on maximum anthocyanin absorption from the crude extract of Cabernet Sauvignon grapes (Vitis vinifera L.) is shown in Figure 1. The same anthocyanin may present different colors, due to pH variations in the solution (Heredia et al., 1998). Control samples presented a bathochromic shift of ∆λ 5.20 with the increase in pH from 1.0 to 4.5. This may have occurred because the existing flavylium cation forms in the crude extract decreased as a function of the pH increase in the solution, causing a slight bathochromic shift in the maximum absorption wavelength (Baranac et al., 1997). Depending on the pH of the solution, the colored flavylium cation co-exists with other forms of anthocyanins, causing the color to fade out quickly. Along with proton transfer reactions, leading to the quinonoidal bases, hydration of flavylium ions gives hemiacetals in equilibrium with chalcones (Berké & Freitas, 2005).
Besides pH, the pigment concentration and the presence of other compounds are factors associated with the anthocyanin-structures detected in solution. In natural sources, anthocyanins are normally associated with colorless compounds (mainly polyphenols and other flavonoids), that have a strong influence upon color (Heredia et al., 1998). In this study, anthocyanin copigmentation reaction was evaluated at pH 1.0, 2.0, 3.0, 3.3, 3.5, 3.7, 4.0 and 4.5 by the addition of organic acids (tannic, gallic, caffeic and ferulic) (1:1 p/v acid/anthocyanin crude extract).
Table 1 shows the values of the bathochromic and hyperchromic shifts obtained after the addition of organic acids to the anthocyanin solutions; these effects characterize copigmentation (Baranac et al., 1997; Dimitric-Markovic et al., 2000; Falcão et al., 2004; Bordignon-Luiz et al., 2006). Since the anthocyanin concentration was constant in all solutions, the magnitude of the bathochromic shift after copigment addition (organic acids) depends only on the presence of acids (Dimitric-Markovic et al., 2000). The addition of tannic, gallic, caffeic and ferulic acid (1:1; p v-1) to anthocyanins solutions from Cabernet Sauvignon grapes (Vitis vinifera L.) at time 0, resulted in increased absorbance (hyperchromic effect) and increased wavelength (bathochromic effect) at every pH value studied, except for caffeic and ferulic acids at pH 1.0 and 2.0, which only showed a bathochromic effect. These hydroxycinnamic acids could increase the formation of pyranoanthocyanins (Gómez-Miguez et al., 2006), hiding the hyperchromic copigmentation effect. The color of pyranoanthocyanins is either orange-red or bluish, depending on the structure. The majority of the naturally occurring pyranoanthocyanins, likely hydroxyphenyl-pyranoanthocyanins, vitisins, and vinylflavanol-pyranoanthocyanins possess a hypsochromically shifted maximum of absorption resulting in orange hues (Rentzsch et al., 2007).
The greatest hyperchromic effect occurred at pH 3.3 with every acid studied. Davies and Mazza (1993) suggested that the copigmentation of anthocyanins with chlorogenic acid, caffeic acid and rutin was also higher between pH 3.3-3.6, where anthocyanins exist essentially as colorless hemiacetals formed due to the hydration of the flavylium cation (Wilska-Jeska and Korzuchowska, 1996). Changes in absorbance values related to pH values, such as those found in Cabernet Sauvignon (Vitis vinifera L.) anthocyanins with and without copigments, could be explained by a mechanism proposed by Brouillard et al. (1991): at pH values between 1 and 2, anthocyanins exist only in the flavylium cation form, and the hyperchromic effect on the maximum wavelength, observed in the presence of a copigment, is due to an interaction between the flavylium cation and the copigments. At pH 3 a waste of anthocyanin color occurs, and significant color retention occurs in anthocyanins with added copigments. So, the role of copigments could consist in inhibiting the pseudobasis formation (colorless) from the flavylium cation. Between pH 4 and 6 anthocyanin solutions remain practically colorless, while the solutions with added copigments still keep their color.
The bathochromic shift, typical in copigmentation reactions, was observed mainly at pH values lower than 3, when a higher concentration of colored forms of anthocyanins occurs. The highest bathochromic and hyperchromic changes observed after ferulic acid addition were similar to those observed by Dimitric-Markovic et al. (2000) for the anthocyanin copigmentation with caffeic and ferulic acids at pH 2.5 and 3.65.
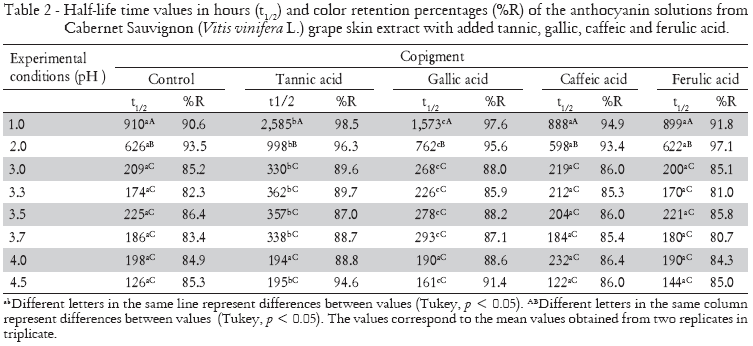
The copigment effect on stability of anthocyanins from the crude extract of Cabernet Sauvignon grapes (Vitis vinifera L.) was followed until 60% or more of the pigments were degraded. The reduction in absorbance values in anthocyanin solutions (pH 1.0 and 4.5) with organic acids over time is presented in Figure 2. Pigments with organic acids kept at pH 1.0 remained steadier when in comparison to those kept at pH 4.5, due to the higher stability of the colored anthocyanin forms. Organic acids improved the stability of our anthocyanin solutions. The half-life time (t1/2) values and color retention percentages (%R) are shown in Table 2.
The use of organic acids and the consequent variation in pH values caused changes in the half-life time of anthocyanins from Cabernet Sauvignon (Vitis vinifera L.) grape crude extract. The increased half-life time (t1/2) of anthocyanins red color after tannic and gallic acids addition were observed at practically all pH values. The addition of tannic acid provided the greatest half-life time for the anthocyanin solutions (2585 h), enhancing the stability by an average of 184% in samples maintained at pH 1.0. Contrasting the copigmenting effects, tannic acid showed differences with the other organic acids (gallic, p = 0.04; caffeic, p = 0.0004; ferulic, p = 0.0001).
Although they both caffeic and ferulic acids caused bathochromic and/or hyperchromic shifts in anthocyanin maximum absorption wavelengths, suggesting an interaction between pigment and copigment, the half-life times revealed no changes (p > 0.05) when compared to control samples. These acids are known for their copigmentation abilities with anthocyanins, which increase red color stability (Dimitric-Markovic et al., 2000; Gris et al., 2007a; Gris et al., 2007b). However, all naturally-occurring hydroxycinnamic acids, e.g., coumaric, caffeic, ferulic, and sinapic acid, can directly react with malvidin-3-glucoside in a chemical reaction without enzymatic catalysis. Thus, pyranoanthocyanins are formed and may have a noticeable impact on the perceived color of wines and juices (Schwarz et al., 2005), modifying absorbance values of copigmented anthocyanins. In this way, the copigmentation effect of caffeic and ferrulic acids in promoting the red color protection of the anthocyanins in crude Cabernet Sauvignon (Vitis vinifera L.) grape skin extract could be hidden by the formation of pyranoanthocyanins.
Color retention ratio was changed by solution pH and copigment addition. However, no significant interaction between the factors was observed (p = 0.999). Anthocyanins solutions with added tannic acid presented the highest percentage of color retention at the end of the tests, due to the prevention of the copigmentation reaction by flavylium cation hydration. In solutions maintained at pH 1.0, color retention percentage was 98.5% after the addition of tannic acid, followed by the addition of gallic (97.6%), caffeic (94.9%) and ferulic acid (91.8%), maintained at the same pH value. At the majority of the times measured, color protection by the copigments at the other pH values occurred in the following order: tannic > gallic > caffeic > ferulic (Table 2). Our results contrasted with those reported by Eiro and Heinonen (2002), who have studied copigmentation reaction in anthocyanins with the caffeic, ferulic, chlorogenic, rosmarinic and gallic acids addition. Eiro and Heinonen (2002) observed that ferulic and caffeic acids addition improves the stability of the anthocyanins colors in a time-dependent manner, and that gallic acid presented a lower capacity of preventing the color fading. To explain part of our results, the formation of pyranoanthocyanins must be studied in future studies.
Conclusion
The copigmentation of anthocyanins from crude Cabernet Sauvignon grape skin extract is influenced by copigment structure and by the pH values at which the reaction occurs. The pH values of the solutions directly influenced the magnitude of the bathochromic and the hyperchromic shifts; meanwhile the organic acid structure contributed for the increase of the half-life of the pigments. Stability of anthocyanins color was higher when tannic acid was added to the system; caffeic and ferulic acid could have increased the formation of pyranoanthocyanins, decreasing the red color of the solutions.
Acknowledgements
The authors are grateful to the Brazilian Government for their financial support via the Conselho Nacional de Pesquisa (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
Association of Official Analytical Chemists [AOAC]. 2005. Official Methods of Analysis. 18ed. AOAC, Washington, D.C., USA.
Awika, J.M. 2008. Behavior of 3-deoxyanthocyanidins in the presence of phenolic copigments. Food Research international 41: 532-538.
Baranac, J.M.; Petranovic, N.A.; Dimitric-Markovic, J.M. 1997. Spectrophotometric study of anthocyan copigmentation reactions. 2. Malvin and the nonglycosidized flavone quercetin.Journal of Agricultural and Food Chemistry 45: 1694-1697.
Berké, B.; Freitas, V.A.P. 2005. Influence of procyanidin structures on their ability to complex with oenin. Food Chemistry 90: 453-460
Bordignon-Luiz, M.T.; Gauche, C.; Gris, E.F.; Falcão, L.D. 2007. Color stability of anthocyanins from Isabel grapes (Vitis labrusca L.) in model systems. Lebensmittel - Wissenschaft und Technologie 40: 594-599.
Brouillard, R.; Wigand, M.C.; Dangles, O.; Cheminat, A. 1991. pH and solvent effect on the copigmentation reaction of malvin with polyphenols, purine and pyrimidine derivatives. Journal of the Chemical Society-Perkin Transactions II 2: 1235-1241.
Davies, A. J.; Mazza, G. 1993. Copigmentation of simple acylated anthocyanins with colorless phenolic compounds. Journal of Agricultural and Food Chemistry 41: 716-720.
Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. 2000. Natural pigments: carotenoids, anthocyanins and betalains- characteristics, biosynthesis, processsing and stability. Critical Reviews in Food and Nutrition 40: 173-289.
Dimitric-Markovic, J.M.; Petranovic, N.A.; Baranac, J.M. 2000. A spectrophotometric study of the copigmentation of malvin with caffeic and ferulic acids. Journal of Agricultural and Food Chemistry 48: 5530-5536.
Dougall, D.K.; Baker, D.C. 2008. Effects of reaction mixture and other components on the determination of the equilibrium and rate constants of the hydration reactions of anthocyanins. Food Chemistry 107: 473-482.
Eiro, M.J.; Heinonen, M. 2002. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. Journal of Agricultural and Food Chemistry 50: 7461-7466.
Falcão, L.D.; Gauche, C.; Barros, D.M.; Prudêncio, E.S.; Gris, E.F.; Sant'Anna, E.S.; Bordignon-Luiz, M.T. 2004. Stability of anthocyanins from grape (Vitis vinifera L.) skins with tannic acid in a model system. Italian Journal of Food Science 16: 323-332.
Fossen, T.; Cabrita, L.; Andersen, O.M. 1998. Colour and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chemistry 63: 435-440.
Fuleki, T.; Francis, F.J.1968. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanins in cranberries. Journal of Food Science 33: 72-77.
Giusti, M.; Saona-Rodriguez, L.E.; Worsltad, R.E. 1999. Molar absortivity and color characteristics of acylated and non-acylated pelargonidin-based anthocyanins. Journal of Agricultural and Food Chemistry 47: 4631-4637.
Giusti, M.M.; Worsltad, R.E. 2001. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy: Current Protocols in Food Analytical Chemistry. John Willey, New York, NY, USA.
Gomez-Míguez, M.; González-Manzano, S.; escribano-Bailón, M.T.; Heredia, F.J.; Santos-Buelga, C. 2006. Influence of different phenolic copigments on the color of malvidin 3-glucoside. Journal of Agricultural and Food Chemistry 54: 5422-5429. Gris, E.F.; Ferreira, E.A.; Falcão, L.D.; Bordignon-Luiz, M.T. 2007a. Caffeic acid copigmentation of anthocyanins from Cabernet Sauvignon grape extracts in model systems. Food Chemistry 100: 1289-1296.
Gris, E.F.; Ferreira, E.A.; Falcão, L.D.; Bordignon-Luiz, M.T. 2007b. Influence of ferulic acid on stability of anthocyanins from Cabernet Sauvignon grapes in a model system and a yogurt system. International Journal of Food Science and Technology 42: 992-998.
Heredia, F.J.; Francia-Aricha, E.M.; Rivas-Gonzalo, J.C.; Vicario, I.M.; Santos-Buelga, C. 1998. Chromatic characterization of anthocyanins from red grapes I. PH effect. Food Chemistry 63: 491-498.
Katsaboxakis, K.; Papanicolaou, D.; Melanitou, M. 1998. Stability of pigmented orange anthocyanins in model and real food system. Italian Journal Food Science 10: 17-25.
Kirca, A.; Cemeroglu, B .2003. Degradation kinetics of anthocyanins in blood orange juice and concentrate. Food Chemistry 81: 583-587.
Lapidot, T.; Harel, S.; Akiri, B.; Granit, R.; Kanner, J. 1999. pH-Dependent forms of red wine anthocyanins as antioxidants. Journal of Agricultural and Food Chemistry 47: 67-70.
Lees, D.H.; Francis, F.G. 1972. Standardization of pigment analysis in cramberries. Hortscience 7: 83-84.
Markakis, P. 1982. Anthocyanin as Food Colors. Academic Press, New York, NY, USA.
Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. 2003. The stomach as a site for anthocyanins absorption from food. Federation of the European Biochemical Societies Letters 544: 210-213.
Rentzsch, M.; Schwarz, M.; Winterhalter, P. 2007. Pyranoanthocyanins : an overview on structures, occurrence and pathways of formation. Trends in Food Science & Technology 18: 526-534.
Schwarz, M.; Picazo-Bacete, J.J.; Winterhalter, P.; Hermosian-Gutiearrez, I. 2005. Effect of copigments and grape cultivar on the color of red wines fermented after the addition of copigments. Journal of Agricultural and Food Chemistry 53: 8372-8381.
Torskangerpoll, K.; Andersen, O.M. 2005. Color stability of anthocyanins in aqueous solutions at various pH values. Food Chemistry 89: 427-440.
Wilska-Jeska, J.; Korzuchowska, A. 1996. Anthocyanins and chlorogenic acid copigmentation: Influence on the color of strawberry and chokeberry juices. Zlebensm Unters Forsch 203: 38-42.
Wulf, L.W.; Nagel, C.W. 1978. High-pressure liquid chromatographic separation of anthocyanins of Vitis vinifera. American Journal of Enology Viticulture 29: 42-49.
Received June 12, 2008
Accepted August 21, 2009
- Association of Official Analytical Chemists [AOAC]. 2005. Official Methods of Analysis. 18ed. AOAC, Washington, D.C., USA.
- Awika, J.M. 2008. Behavior of 3-deoxyanthocyanidins in the presence of phenolic copigments. Food Research international 41: 532-538.
- Baranac, J.M.; Petranovic, N.A.; Dimitric-Markovic, J.M. 1997. Spectrophotometric study of anthocyan copigmentation reactions. 2. Malvin and the nonglycosidized flavone quercetin.Journal of Agricultural and Food Chemistry 45: 1694-1697.
- Berké, B.; Freitas, V.A.P. 2005. Influence of procyanidin structures on their ability to complex with oenin. Food Chemistry 90: 453-460
- Bordignon-Luiz, M.T.; Gauche, C.; Gris, E.F.; Falcão, L.D. 2007. Color stability of anthocyanins from Isabel grapes (Vitis labrusca L.) in model systems. Lebensmittel - Wissenschaft und Technologie 40: 594-599.
- Brouillard, R.; Wigand, M.C.; Dangles, O.; Cheminat, A. 1991. pH and solvent effect on the copigmentation reaction of malvin with polyphenols, purine and pyrimidine derivatives. Journal of the Chemical Society-Perkin Transactions II 2: 1235-1241.
- Davies, A. J.; Mazza, G. 1993. Copigmentation of simple acylated anthocyanins with colorless phenolic compounds. Journal of Agricultural and Food Chemistry 41: 716-720.
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. 2000. Natural pigments: carotenoids, anthocyanins and betalains- characteristics, biosynthesis, processsing and stability. Critical Reviews in Food and Nutrition 40: 173-289.
- Dimitric-Markovic, J.M.; Petranovic, N.A.; Baranac, J.M. 2000. A spectrophotometric study of the copigmentation of malvin with caffeic and ferulic acids. Journal of Agricultural and Food Chemistry 48: 5530-5536.
- Dougall, D.K.; Baker, D.C. 2008. Effects of reaction mixture and other components on the determination of the equilibrium and rate constants of the hydration reactions of anthocyanins. Food Chemistry 107: 473-482.
- Eiro, M.J.; Heinonen, M. 2002. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. Journal of Agricultural and Food Chemistry 50: 7461-7466.
- Falcão, L.D.; Gauche, C.; Barros, D.M.; Prudêncio, E.S.; Gris, E.F.; Sant'Anna, E.S.; Bordignon-Luiz, M.T. 2004. Stability of anthocyanins from grape (Vitis vinifera L.) skins with tannic acid in a model system. Italian Journal of Food Science 16: 323-332.
- Fossen, T.; Cabrita, L.; Andersen, O.M. 1998. Colour and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chemistry 63: 435-440.
- Fuleki, T.; Francis, F.J.1968. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanins in cranberries. Journal of Food Science 33: 72-77.
- Giusti, M.; Saona-Rodriguez, L.E.; Worsltad, R.E. 1999. Molar absortivity and color characteristics of acylated and non-acylated pelargonidin-based anthocyanins. Journal of Agricultural and Food Chemistry 47: 4631-4637.
- Giusti, M.M.; Worsltad, R.E. 2001. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy: Current Protocols in Food Analytical Chemistry. John Willey, New York, NY, USA.
- Gomez-Míguez, M.; González-Manzano, S.; escribano-Bailón, M.T.; Heredia, F.J.; Santos-Buelga, C. 2006. Influence of different phenolic copigments on the color of malvidin 3-glucoside. Journal of Agricultural and Food Chemistry 54: 5422-5429.
- Gris, E.F.; Ferreira, E.A.; Falcão, L.D.; Bordignon-Luiz, M.T. 2007a. Caffeic acid copigmentation of anthocyanins from Cabernet Sauvignon grape extracts in model systems. Food Chemistry 100: 1289-1296.
- Gris, E.F.; Ferreira, E.A.; Falcão, L.D.; Bordignon-Luiz, M.T. 2007b. Influence of ferulic acid on stability of anthocyanins from Cabernet Sauvignon grapes in a model system and a yogurt system. International Journal of Food Science and Technology 42: 992-998.
- Heredia, F.J.; Francia-Aricha, E.M.; Rivas-Gonzalo, J.C.; Vicario, I.M.; Santos-Buelga, C. 1998. Chromatic characterization of anthocyanins from red grapes I. PH effect. Food Chemistry 63: 491-498.
- Katsaboxakis, K.; Papanicolaou, D.; Melanitou, M. 1998. Stability of pigmented orange anthocyanins in model and real food system. Italian Journal Food Science 10: 17-25.
- Kirca, A.; Cemeroglu, B .2003. Degradation kinetics of anthocyanins in blood orange juice and concentrate. Food Chemistry 81: 583-587.
- Lapidot, T.; Harel, S.; Akiri, B.; Granit, R.; Kanner, J. 1999. pH-Dependent forms of red wine anthocyanins as antioxidants. Journal of Agricultural and Food Chemistry 47: 67-70.
- Lees, D.H.; Francis, F.G. 1972. Standardization of pigment analysis in cramberries. Hortscience 7: 83-84.
- Markakis, P. 1982. Anthocyanin as Food Colors. Academic Press, New York, NY, USA.
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. 2003. The stomach as a site for anthocyanins absorption from food. Federation of the European Biochemical Societies Letters 544: 210-213.
- Rentzsch, M.; Schwarz, M.; Winterhalter, P. 2007. Pyranoanthocyanins : an overview on structures, occurrence and pathways of formation. Trends in Food Science & Technology 18: 526-534.
- Schwarz, M.; Picazo-Bacete, J.J.; Winterhalter, P.; Hermosian-Gutiearrez, I. 2005. Effect of copigments and grape cultivar on the color of red wines fermented after the addition of copigments. Journal of Agricultural and Food Chemistry 53: 8372-8381.
- Torskangerpoll, K.; Andersen, O.M. 2005. Color stability of anthocyanins in aqueous solutions at various pH values. Food Chemistry 89: 427-440.
- Wilska-Jeska, J.; Korzuchowska, A. 1996. Anthocyanins and chlorogenic acid copigmentation: Influence on the color of strawberry and chokeberry juices. Zlebensm Unters Forsch 203: 38-42.
- Wulf, L.W.; Nagel, C.W. 1978. High-pressure liquid chromatographic separation of anthocyanins of Vitis vinifera. American Journal of Enology Viticulture 29: 42-49.
Publication Dates
-
Publication in this collection
16 Mar 2010 -
Date of issue
Feb 2010
History
-
Accepted
21 Aug 2009 -
Received
12 June 2008