Abstracts
Plant wastes present a high cellulose content, which is an ideal organic material for composting. Five strains of thermophiles from processed Brassica waste were isolated, and the hydrolytic activity on various cellulosic biomass substrata and their temperature profiles were determined. 16S rRNA sequencing identified these strains as Thermoactinomyces and Bacillus spp. Maximal cellulase activity corresponded to 2.3 U mL-1 of enzyme. The application of these strains on Brassica rapa residues demonstrates increased total nitrogen content). TA-3, a Thermoactinomycetes sp. strain, performs best among all inoculants, increasing the nitrogen content from 0.74 to 0.91%, and decreasing the carbon content from 15.4 to 12.2%, showing its high efficiency and bioactivity during compositing.
cellulose degrading; enzymes; vegetable wastes
Resíduos vegetais apresentam alta concentração de celulose, que é um material orgânico ideal para preparação de composto. Cinco linhagens de termófilos foram isoladas de resíduos processados de Brassica e a atividade hidrolítica em vários substratos contendo celulose e seus padrões de temperatura foram determinados. O seqüenciamento de rRNA 16S permitiu a identificação dessas isolados como Thermoactinomyces e Bacillus spp. A atividade máxima de celulase foi determinada como de 2,3 U mL-1 de enzima. O uso dessas linhagens em resíduos de Brassica rapa resultou em um aumento total do conteúdo de nitrogênio. TA-3, uma linhagem de Thermoactinomycetes sp., apresentou melhor desempenho entre os inoculantes, aumentando o conteúdo de nitrogênio de 0,74 para 0,91%, e diminuindo o conteúdo de carbono de 15,4 para 12,2%, mostrando sua alta eficiência e bioatividade durante a compostagem.
degradação de celulose; enzimas; resíduos vegetais
GENETICS AND PLANT BREEDING
Activity of cellulase from Thermoactinomycetes and Bacillus spp. isolated from Brassica waste compost
Atividade de celulase de Termoactinomicetos e Bacillus spp. isolados de resíduos derivados de compostos de Brassica
Chen-Chin ChangI,* * Corresponding author < t10009@mail.tut.edu.tw> ; Chang-Chai NgII; Chung-Yi WangII; Yuan-Tay ShyuII
ITainan University of Technology - Dept. of Home Science - 529 Jhongjheng Rd. - Yongkang, Tainan - 71002 - Taiwan ROC
IINational Taiwan University - Dept. of Horticulture - 140 Keelung Rd. - Section 4 - Taipei - 106 - Taiwan ROC
ABSTRACT
Plant wastes present a high cellulose content, which is an ideal organic material for composting. Five strains of thermophiles from processed Brassica waste were isolated, and the hydrolytic activity on various cellulosic biomass substrata and their temperature profiles were determined. 16S rRNA sequencing identified these strains as Thermoactinomyces and Bacillus spp. Maximal cellulase activity corresponded to 2.3 U mL-1 of enzyme. The application of these strains on Brassica rapa residues demonstrates increased total nitrogen content). TA-3, a Thermoactinomycetes sp. strain, performs best among all inoculants, increasing the nitrogen content from 0.74 to 0.91%, and decreasing the carbon content from 15.4 to 12.2%, showing its high efficiency and bioactivity during compositing.
Key words: cellulose degrading, enzymes, vegetable wastes
RESUMO
Resíduos vegetais apresentam alta concentração de celulose, que é um material orgânico ideal para preparação de composto. Cinco linhagens de termófilos foram isoladas de resíduos processados de Brassica e a atividade hidrolítica em vários substratos contendo celulose e seus padrões de temperatura foram determinados. O seqüenciamento de rRNA 16S permitiu a identificação dessas isolados como Thermoactinomyces e Bacillus spp. A atividade máxima de celulase foi determinada como de 2,3 U mL-1 de enzima. O uso dessas linhagens em resíduos de Brassica rapa resultou em um aumento total do conteúdo de nitrogênio. TA-3, uma linhagem de Thermoactinomycetes sp., apresentou melhor desempenho entre os inoculantes, aumentando o conteúdo de nitrogênio de 0,74 para 0,91%, e diminuindo o conteúdo de carbono de 15,4 para 12,2%, mostrando sua alta eficiência e bioatividade durante a compostagem.
Palavras-chaves: degradação de celulose, enzimas, resíduos vegetais
INTRODUCTION
Agricultural waste generated in field and processing sites is generally discarded without being further used. If this waste is not properly handled, it may create a large environmental burden. Approximately 4.2 million metric tons of agricultural waste is produced annually in Taiwan (COA, 2006). Yang (1997) stated that fruit and vegetables account for almost 15% of total waste produced, comprising 570,000 metric tons of matter with high cellulose content. This waste can be handled in several ways, including direct combustion, landfill and composting. Among these methods, composting is considered the optimum approach for regulating soil fertility and minimizing the environmental impact.
Cellulose is the most common carbohydrate occurring in plants throughout the world. In fruit and vegetables, cellulose comprises almost 50% of carbohydrate, while hemi-cellulose comprises 15-34% (Das & Singh, 2004 and Reddy & Young, 2005). Most of these compounds are difficult to decompose rapidly in the natural environment (Knauf & Moniruzzaman, 2004 and Reddy & Young, 2005). Various microorganisms isolated from compost have been identified (McCaig et al., 2001; Song et al., 2001 and Das & Singh, 2004). Interest in these microorganisms has increased owing to potential commercial applications, namely biodegradation and production of bioactive compounds, including antibiotics and enzymes (Malherbe & Cloete, 2002; Kirk et al, 2002; Lynd et al., 2005). The composting process used for decomposing vegetable and fruit waste can be accelerated by adding cellulose-production thermophile. Organic matter in soil crucially influences soil systems and increases crop productivity. The application of compost in soil provides a key source of plant nutrients, and improves soil fertility, aeration, porosity, structure and water-holding capacity (Tuomela et al., 2000). With regard to natural systems, soil organic matter management is also essential for sustainable use of agricultural system, and for enhancing soil quality (Malherbe & Cloete, 2002).
Brassica rapa L. var. chinensis, also known as 'Pak-Choi', is one of the most popular vegetables among Chinese. Owing to the harvesting and sorting processes, over a thousand metric tons of waste are discarded annually in Taiwan. In this study, compost was produced from unwanted plant residue gathered from processing factories. Several strains of thermophilic microorganisms were then isolated from the compost and identified via 16S rRNA sequencing. The collected strains were tested for cellulase activity and temperature stability. The screened strains were then further applied to Brassica rapa residue to measure their ability to accelerate the composting process.
MATERIAL AND METHODS
A packed-bed reactor was adopted for making compost (Chang et al., 2004a and Chang et al., 2004b). Raw material such as unwanted outer leaf and stem of Pak-Choi were collected from a processing factory in Changhwa county (24°5' N, 120°32' E). 70% (w w-1) of water content from Pak-Choi was removed using drying chamber at temperature of 45°C for 8 h. The material was then mixed with rice husk (water content 10%) using a ratio of 5 to 1. Aeration was applied from the bottom of the reactor at a rate of 1.71 air kg-1 dry solids min-1. The entire composting process terminated after 90 d. Isolation of microorganism and determination of cellulase activity was undertaken during day 45. Ten to fifty g of compost sample was taken from the compost, and 50 mL sterile water was added. After shaking for 2 h under 200 rpm at 50°C, 1 mL of suspension was serially diluted and plated on a tryptic soy agar, modified M3 agar and potato dextrose agar (Huck et al., 1991).
Plates were incubated at 37°C and 50°C for 7 d. Isolates appeared on the plate were selected and plated on nutrient agar, bacterial selection agar, actinomycetes selection agar and fungal selection agar (Hagerman et al., 1985; Chang et al., 2004a and Chang et al., 2004b). Colonies appeared on selective agar were further used for cellulase activity testing. Cellulase activity was tested on Mandels-Reese agar (Bhat & Wood, 1988; Chang et al., 2004a). Briefly, isolate was plated on Mandels-Reese and cultivated at 50°C for 4 d, and 0.1% of Congo red was then sprayed on the colony. The diameter of the observed clear zone was then recorded.
Extraction of cellulolytic enzymes was referred to Zhang et al. (2006) with little modification. Cellulase-containing crude enzyme was prepared by centrifugation of culture broth at a rate of 12,000 x g and 4°C for 1 h. The supernatant was then filtered using 0.2 mm filter paper. Dinitrosalicylic acid (DNS) method (Miller, 1959) was applied to determine of cellulase activity. The DNS was prepared as described by Bhat & Wood (1988). Carboxymethylcellulose (CMC) was used as the substrate (Damaso et al., 2003). Glucose standard was used to plot the standard curve. Measurement of glucose released from CMC was expressed as Unit (U), in which, U = mg of glucose equivalents released min-1 mL-1 crude enzyme
The genomic DNA of the samples was extracted by method described by Ng et al. (2006). The total 16S rRNA was amplified by primers BSF8 (52 -AGAGTTTGATCCTGGC TCAG-32 ) and BSF1507 (52 -TACCTTGTTACGACTT-32 ) (Ribosomal Database Project II). The PCR products were purified using a Gel Extraction kit, and subsequently ligated into pGEM®-T Vector Systems. The ligation product was transformed into an ECOS® competent cell screened using blue-white screening with IPTG and X-Gal. White colonies were selected and plated on an Luria-Bertani (LB) agar plate (bacto-tryptone 1%, yeast extract 0.5%, agar 1%) containing 50 μg mL-1 Ampicilin. 16S rRNA inserts were then amplified from the colonies by primers TAF (52 -CAAGGCGATTAAGTTGGGTA-32 ) and TAR (5-GGAATTGTGAGCGATAACA-32 ) provided by the pGEM®-T Vector Systems. The amplified fragments were sequenced. Strains isolated were applied on Brassica rapa residue. Inoculation 1 × 108 cell g-1 dry solid was performed, and the total duration of the composting process was 60 d. The composition of the plant residues was then analyzed. The total nitrogen level was tested by the Micro-Kjeldahl method (Singh & Pradhan, 1981). The total organic carbon (TOC) was estimated with Walkey and Black's Rapid Titration method (1934). Levels of cellulose, hemicellulose and lignin were measured using the method described by Dutta (1981).1
RESULTS AND DISCUSSION
Five strains were screened, namely from Thermoactinomycetes and Bacillus, with 16S rRNA similarity in the range 95-99%, following BLAST comparison in the NCBI database. The microorganisms were primarily mesophilic or thermophilic (Table 1). Actinomycetes comprise a diverse group of largely mycelial bacteria, many of which are ecologically important and have commercial potential for enzyme and antibiotic production (Edwards, 2007). Actinomycetes can degrade some cellulose, and solubilize lignin, and tolerate higher temperatures and pH than fungi. Actinomycetes thus are important agents of lignocellulose degradation during peak heating, among which Thermoactinomycetes is one of the genus that appear mainly during the cooling and maturation phase of composting (Tuomela et al., 2000). Previous assessment of soil diversity based on 16S rRNA has facilitated improved understanding of microbial diversity (Baker & Cowan, 2004), this universal and domain-specific region is molecule ubiquitous has numerous molecules, and also reflects functional constancy and is experimentally tractable (Woese, 2000).
Cellulose degradation is caused by various microorganisms, and represents a major carbon flow atmospheric CO2 to fixed carbon. Fixed carbon has considerable potential for use in producing sustainable biobased products (Schloss et al., 2005). Results obtained by molecular systematic require further examination using other biochemical kits or tool because of the existence of some harmful microbes, such as Thermoactinomyces vulgaris, which in addition to their amylolytic activity are also causative agents in lung disease (Allen & Hartman, 1972).
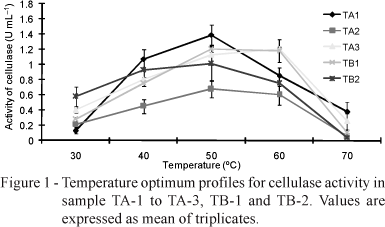
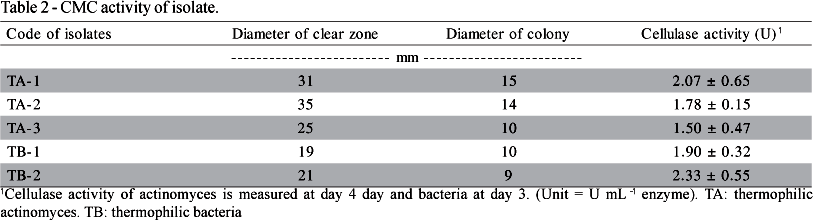
Generally, Actinomycetes and Bacillus strains isolated in this study released 2.0 mg glucose equivalents min-1 mL-1 enzyme (Table 2). These results were similar to those of Mayende et al. (2006), who found that Bacillus from compost exhibited cellulase activity. Numerous past studies have found the presence of thermophilic or thermo-tolerant Bacillus and Actinomycetes in compost (Blanc et al., 1999; Dees & Ghiorse, 2001; McCaig et al., 2001 and Mayende et al., 2006). Mayende et al. (2006) took compost samples from garden refuse, and identified screened microorganisms that were primarily thermophilic (60°C) or extremely thermophilic (70°C), with maximum cellulose activity of between 1.215-1.333 U. Five of the isolates identified in this work could utilize hydroxyethyl-cellulose and β-glucan. Thermoactinomycetes sp. (TA-1) was found to be able to utilize all the cellulose-containing materials (Table 3). A temperature of 50°C was recorded on day 45, demonstrating that most isolated strains were actively involved in the cellulose degradation process. Figure 1 illustrates the optimal temperatures of the isolates. All enzymes worked better at temperatures of 50°C, while those from TA-3 performed best at 60°C. The hydrolysis of CMC could be performed in 60-70 min. The dynamic of the enzymatic process reduced following 70 min for TA-2 and TB-1 (Figure 2).
Table 4 lists changes in nitrogen and carbon content in plant residue inoculated using different isolated strains. The Total Organic Carbon (TOC) of all five inoculants reduced, revealing mineralization of organic matter. Additionally, the Total Kjeldahl nitrogen (TKN) content of all inoculants increased. Notably, TA-3, a Thermoactinomycetes sp. performed best among all inoculants, with nitrogen content increasing from 0.7% to 0.9%, and carbon content decreasing from 15.4% to 12.2% (Table 4). In cellulose content degradation, TA-3 performed best among all inoculants, which from 38.1% decreased to 15.2%. Overall performance of Thermoactinomyces on cellulose, hemicellulose and lignin were found better than those of thermophilic Bacillus. This result resembled the findings of Singh & Sharma (2002), who studied the impact of composting acceleration on bioinoculant efficiency. Non-inoculated wastes exhibited increased TKN and decreased TOC. Cellulose was considerably reduced following inoculation (Table 4). The content of the three organic components, cellulose, hemicellulose and lignin, reduced during composting with maximum degradation using TA-3 inoculation (Table 4). Similar results were also described by Rasal et al. (1988), who adopted cellulolytic fungi to decompose sugarcane trash.
This investigation has further confirmed the wide spread of Bacillus in natural composting systems. It also provides a feasible method of handling the large annual production of cellulose-containing substances. This study also further demonstrates that thermophiles isolated from a composting environment could produce thermophilic cellulases, which have potential industrial applications.
Received July 30, 2007
Accepted August 25, 2008
- ALLEN, M.J.; HARTMAN, P.A. Amylolytic isoenzymes of Thermoactinomyces vulgaris Journal of Bacteriology, v.109, p.452-454, 1972.
- BAKER, G.C.; COWAN, D.A. 16S rDNA primers and the unbiased assessment of thermophile diversity. Biochemical Society Transactions, v.32, p.218-221, 2004.
- BHAT, K.M.; WOOD, T.M. Heterogeneity of endo-(1-4)-beta D-glucanase activity in Penicillium pinophilum cellulase. Biochemical Society Transactions, v.17, p.104-105, 1988.
- BLANC, M.; MARILLEY, L.; BEFFA, T.; ARAGNO, M. Thermophilic bacterial communities in hot composts as revealed by most probable number counts and molecular (16S rDNA) methods. FEMS Microbiology Ecology, v.28, p.141-149, 1999.
- CHANG, C.C.; NG, C.C.; SHYU, Y.T. Screening of cellulose decomposition microorganisms from cauliflower waste compost. Journal of the Agriculture Association of China, v.5, p.551-559, 2004a.
- CHANG, C.C., TZENG, W.C., SHYU, Y.T. Effects of Bacillus subtilis treated compost on growth of Brassica rapa L. var. Chinese Journal of the Chinese Society of Horticultural Science, v.50, p.337-342, 2004b.
- COUNCIL OF AGRICULTURE - COA. Agricultural Yearbook. Taipei: COA Press, 2006. 266p.
- DAMASO, M.C.T.; ALMEIDA, M.S.; KURTENBACH, E.; MARTINS, O.B.; PEREIRA, N.; ANDRADE, C.M.M.C.; ALBANO, R.M. Optimized expression of a thermostable xylanase from Thermomyces lanuginosus in Pichia pastoris Applied & Environmental Microbiology, v.69, p.6064-6072, 2003.
- DAS, H.; SINGH, S.K. Useful byproducts from cellulosic wastes of agriculture and food industry-a critical appraisal. Critical Revision on Food Science and Nutrition, v.44, p.77-89, 2004.
- DEES, P.M.; GHIORSE, W.C. Microbial diversity in hot synthetic compost as revealed by PCR-amplified rRNA sequences from cultivated isolates and extracted DNA. FEMS Microbiology Ecology, v.35, p.207-216, 2001.
- DUTTA, R. Acidogenic fermentation of lignocellulosic acid yield and conversion of components. Biotechnology & Bioengineering, v.23, p.2167-2170, 1981.
- EDWARDS, C. Isolation properties and potential applications of thermophilic actinomycetes. Applied Biochemistry and Biotechnology, v.42, p.161-179, 2007.
- HAGERMAN, A.E.; BLAU, D.H.; McLURE, A.L. Plate assay for determining the time of production of protease, cellulase and pectinases by germinating fungal spores. Analytical Biochemistry, v.151, p.334-42, 1985.
- HUCK, T.A.; PORTER, N.; BUSHELL, M.E. 1991. Positive selection of antibiotic-producing soil isolates. Journal of General Microbiology, v.137, p.2321-2329, 1991.
- KIRK, O.; BORCHERT, T.V.; FUGLSANG, C.C. Industrial enzyme applications. Current Opinion in Biotechnology, v.13, p.345-351, 2002.
- KNAUF, M.; MONIRUZZAMAN, M. Lignocellulosic biomass processing: a perspective. International Sugar Journal, v.106, p.47-150, 2004
- LYND, L.R.; ZYL, W.H. van; McBRIDE, J.E.; LASER, M. Consolidated bioprocessing of cellulosic biomass: an update. Current Opinion in Biotechnology, v.16, p.577-583, 2005.
- MALHERBE, S.; CLOETE, T.E. Lignocellulose biodegradation: fundamentals and applications. Reviews in Environmental Science & Biotechnology, v.1, p.105-114, 2002.
- MAYENDE, L.; BRENDAN S.W.; BRETT I.P. Cellulases (CMCases) and polyphenol oxidases from thermophilic Bacillus spp. isolated from compost. Soil Biology & Biochemistry, v.38, p.2963-2966, 2006.
- McCAIG, A.E.; GRAYSTON, S.J.; PROSSER, J.I.; GLOVER, L.A. Impact of cultivation on characterisation of species composition of soil bacterial communities. FEMS Microbiology Ecology, v.35, p.37-48, 2001.
- MILLER, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, v.31, p.426-428, 1959.
- NG, C.C.; HUANG, W.C.; CHANG, C.C.; TZENG, W.S.; CHEN, T.W.; LIU, Y.S.; SHYU, Y.T. Tufa microbial diversity revealed by 16S rRNA cloning in Taroko National Park, Taiwan. Soil Biology & Biochemistry, v.38, p.342-348, 2006.
- RASAL, P.H.; KALBHAR, H.B.; SHINGTE, V.V.; PATIL, P.L. Development of technology for rapid composting and enrichment. In: SEN, S.P.; PALIT, P. (Ed.) Biofertilizers: potentialities and problems. Calcutta: Plant Physiology Forum and Naya Prakash, 1988. p.255-258.
- REDDY, N.; YANG, Y. Biofibers from agricultural by products for industrial applications. Trends in Biotechnology, v.23, p.22-27, 2005.
- SCHLOSS, P.D.; HAY, A.G.; WILSON, D.B.; GOSSETT, J.M., WALKER, L.P. Quantifying bacterial population dynamics in compost using 16S rRNA gene probes. Applied Microbiology and Biotechnology, v.66, p.457-463, 2005.
- SINGH, R.; PRADHAN, K. Determination of nitrogen and protein by Kjeldahl method: forage evaluation science. New Delhi: Pvt, 1981. 23p.
- SINGH, A.; SHARMA, S. Composting of a crop residue through treatment with microorganisms and subsequent vermicomposting. Bioresource Technology, v.85, p.107-111, 2002
- SONG, J.; WEON, H.Y.; YOON, S.H.; PARK, D.S.; GO, S.J.; SUH, T.W. Phylogenetic diversity of thermophilic actinomycetes and Thermoactinomyces spp. isolated from mushroom composts in Korea based on 16S rRNA gene sequence analysis. FEMS Microbiology Letters, v.202, p.97-102, 2001.
- TUOMELA, M.; VIKMAN, M.; HATAKKA, A.; ITAVAARA, M. Biodegradation of lignin in a compost environment: a review. Bioresource Technology, v.72, p.169-183, 2000.
- WALKEY, J.A.; BLACK, J.A. Estimation of organic carbon by the chromic acid titration method. Soil Science, v.37, p.29-31, 1934.
- WOESE, C.R. Interpreting the universal phylogenetic tree. Proceedings of the National Academy of Sciences, v.97, p.8392-8396, 2000.
- YANG, S.S. Preparation and characterization of compost. Journal of the Biomass Energy Society of China, v.16, p.47-62, 1997.
- ZHANG, Y.H.; HIMMEL, M.E; MIELENZ, J.R. Outlook for cellulase improvement: Screening and selection strategies. Biotechnology Advances, v.24, p.452-481, 2006.
Publication Dates
-
Publication in this collection
29 June 2009 -
Date of issue
June 2009
History
-
Received
30 July 2007 -
Accepted
25 Aug 2008