Abstract
Abstract: This study aimed to evaluate the in vitro antiproliferative and inhibition of oxidative DNA-damage activities of n-butanol (n-BuOH) extract of Centaurea sphaerocephala. The in vitro antioxidant activity of the ethyl acetate (EtOAc) and the n-BuOH extracts of this plant were also assayed. To investigate the antioxidant potential, extracts were tested for their capacity to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH·) and to inhibit lipid peroxidation using the TBARs method. The contents of total phenolics and flavonoids were measured. Additionally, antiproliferative activity and DNA-damage inhibition of the n-BuOH extract was determined using XCELLigence RTCA instrument and photolyzing 46966 plasmid, respectively. The results exhibited that the scavenging abilities of the EtOAc extract were better than the n-BuOH extract with an IC50= 11.59 µg/mL and 16.67 µg/mL for both extracts, respectively. The phenolic and flavonoid contents were found higher in the n-BuOH and EtOAc extracts. Furthermore, our results showed that n-BuOH extract exhibited a remarkable inhibition of lipid peroxidation with an IC50 of 340.94±7.49 μg/mL and had an antiproliferative effect against Hela cells. Extracts of C. sphaerocephala showed antioxidant activity on scavenging DPPH·. In addition, the n-BuOH extract inhibited the lipid peroxidation and exhibited an antiproliferative effect against HeLa cells line (human cervix carcinoma).
Key words
Centaurea sphaerocephala; phenolics; antioxidant; lipid peroxidation; antiproliferative; DNA-damaged inhibition
INTRODUCTION
Plants produce an excessive number of antioxidants to represent a potential source of new molecules and prevent oxidative stress for therapeutic uses (CraggCRAGG GM and NEWMAN DJ. 2013. Natural products: a continuing source of novel drug leads. BBA-GEN Subjects 1830: 3670-3695. and Newman 2013). Some medicinal plants have noteworthy potential as natural antioxidants and may be used as protectors against disorders related to oxidative stress (TingTING H-C, HSU Y-W, TSAI C-F, LU F-J, CHOU M-C and CHEN W-K. 2011. The in vitro and in vivo antioxidant properties of seabuckthorn (Hippophae rhamnoides L.) seed oil. Food Chem 125: 652-659. et al. 2011, BoraBORA KS and SHARMA A. 2011. Evaluation of antioxidant and free-radical scavenging potential of Artemisia absinthium. Pharm Biol 49: 1216-1223. and Sharma 2011). However, increasing needs for natural antioxidants have provoked great importance for the discovery of powerful free radical scavengers from plants (KhodaieKHODAIE L, BAMDAD S, DELAZAR A and NAZEMIYEH H. 2012. Antioxidant, total phenol and flavonoid contents of two pedicularis l. species from Eastern Azerbaijan, Iran. Bioimpacts: BI 2: 43-57. et al. 2012). Some plants showed a radical scavenging capacity dose-dependently and a protective effect on H2O2-induced cytotoxicity and DNA damage (RussoRUSSO A, IZZO A, CARDILE V, BORRELLI F and VANELLA A. 2001. Indian medicinal plants as antiradicals and DNA cleavage protectors. Phytomedicine 8: 125-132. et al. 2001). The olive leaf extract, known for its antioxidant capacity, has been confirmed to induce apoptosis in several cancer cells via differentiation steps (SametSAMET I, HAN J, JLAIEL L, SAYADI S and ISODA H. 2014. Olive (Olea europaea) leaf extract induces apoptosis and monocyte/macrophage differentiation in human chronic myelogenous leukemia K562 cells: insight into the underlying mechanism. Oxid Med Cell Longev 2014: 1-17. et al. 2014). Moreover, Boerhavia elegans (Choisy) exhibited strong anti-malarial effect and antioxidant activity due to its high content of phenolic components (SadeghiSADEGHI Z, VALIZADEH J, SHERMEH OA and AKABERI M. 2015. Antioxidant activity and total phenolic content of Boerhavia elegans (choisy) grown in Baluchestan, Iran. Avicenna J Phytomed 5: 1-9. et al. 2015). The genus Centaurea (trible Cynareae, family Asteraceae) is one of the most widely distributed plant genera in the world. Centaurea includes more than 500 species, 45 of which grow spontaneously in Algeria, with 7 species localized in the Sahara region (OzendaOZENDA P. 1958. Flore du Sahara Septentrional et Central. Centre National de la Recherche Scientifique(CNRS), Paris, p. 450-454. 1958, QuézelQUÉZEL P and SANTA S. 1963. Nouvelle flore de l’Algérie et des régions désertiques méridionales: Centre National de La Recherche Scientifique (CNRS) Paris, Tome II., 1963, p 1032. and Santa 1963). Several Centaurea species are consumed in folk medicine in many countries. For example in Turkey, dried flowers of Centaurea cyanus are used as an infusion to relieve diarrhea, gain energy and increase appetite; infusion of Centaurea calcitrapa is used as a febrifuge; Centaurea jacea is used to increase appetite, to relieve constipation, reduce fever and start menstruation (ReyhanREYHAN A, KÜPELİ E and ERGUN F. 2004. The biological activity of Centaurea L. species. Gazi U J Sci 17: 149-164. et al. 2004, BaytopBAYTOP T. 1999. Türkiye’de Bitkiler ile Tedavi (Geçmiste ve Bugün) İlaveli Ikinci Baskı. Nobel Tıp Kitabevleri, (No:3255), Istanbul (in Turkish), p. 316. 1999). In Tunisia, Centaurea furfuracea, an endemic species from the desert region of the North of Africa, is used as astringent and diuretic (FakhfakhFAKHFAKH JAE and DAMAK M. 2007. Sesquineolignans from the flowers of Centaurea furfuracea Coss. et Dur.(Asteraceae). Nat Prod Res 21: 1037-1041. and Damak 2007). While in Algeria, the roots of Centaurea incana are used in the region of Aurès for the treatment of liver diseases (AclinouACLINOU P, BOUKERB A, BOUQUANT J, MASSIOT G and MEN-OLIVER L. 1982. Plantes des Aures: constituants des racines de Centaurea incana (Composes). Plant Med Phytother 16: 303-309. et al. 1982).
In addition, various studies have shown medicinal properties of Centaurea species such as antimicrobial (KariotiKARIOTI A, SKALTSA H, LAZARI D, SOKOVIC M, GARCIA B and HARVALA C. 2002. Secondary metabolites from Centaurea deusta with antimicrobial activity. Z Naturforsch C 57: 75-80. et al. 2002), antibacterial (ĆirićĆIRIĆ A, KARIOTI A, GLAMOČLIJA J, SOKOVIĆ M and SKALTSA H. 2011. Antimicrobial activity of secondary metabolites isolated from Centaurea spruneri Boiss. & Heldr. J Serb Chem Soc 76: 27-34. et al. 2011), antifungal (KoukoulitsaKOUKOULITSA C, GEROMICHALOS GD and SKALTSA H. 2005. VolSurf analysis of pharmacokinetic properties for several antifungal sesquiterpene lactones isolated from Greek Centaurea sp. J Comput Aid Mol Des 19: 617-623. et al. 2005), cytotoxic (Koukoulitsa et al. 2002KOUKOULITSA E, SKALTSA H, KARIOTI A, DEMETZOS C and DIMAS K. 2002. Bioactive sesquiterpene lactones from Centaurea species and their cytotoxic/cytostatic activity against human cell lines in vitro. Planta Med 68: 649-652., TukovTUKOV FF, ANAND S, GADEPALLI RSV, GUNATILAKA AL, MATTHEWS JC and RIMOLDI JM. 2004. Inactivation of the cytotoxic activity of repin, a sesquiterpene lactone from Centaurea repens. Chem Res Toxicol 17: 1170-1176. et al. 2004) and analgesic (Hamid Oudjana 2017). Centaurea is well known for its high structural diversity in major bioactive structures, including sesquiterpene lactones, lignans, triterpene and flavonoids (Koukoulitsa et al. 2002, 2005, Tukov et al. 2004, Hamid Oudjana 2017).
Given the interest of Centaurea pharmacology and phytochemistry and within the context of the study of Centaurea species growing in Algeria ZaterZATER H, HUET J, FONTAINE V, BENAYACHE S, STÉVIGNY C, DUEZ P and BENAYACHE F. 2016. Chemical constituents, cytotoxic, antifungal and antimicrobial properties of Centaurea diluta Ait. subsp. algeriensis (Coss. & Dur.) Maire. Asian Pac J Trop Med 9: 554-561. et al. 2016, KolliKOLLI EH, LEÓN F, BENAYACHE F, ESTÉVEZ S, QUINTANA J, ESTÉVEZ F, BROUARD I, BERMEJO J and BENAYACHE S. 2012. Cytotoxic sesquiterpene lactones and other constituents of Centaurea omphalotricha. J Braz Chem Soc 23: 977-983. et al. 2012, BichaBICHA S, BENTAMENE A, BENAISSA O, BENAYACHE S, GARCIA V, LEON F, BROUARD I, BERMEJO J and BENAYACHE F. 2011. Flavonoid aglycones from Centaurea maroccana. Chem Nat Compd 47: 105-106. et al. 2011, BentameneBENTAMENE A, BAZ M, BOUCHEHAM R, BENAYACHE S, CRECHE J and BENAYACHE F. 2008. Flavonoid aglycones from Centaurea sphaerocephala. Chem Nat Compd 2: 234-235. et al. 2010, MedjroubiMEDJROUBI K, BENAYACHE F and BERMEJO J. 2005. Sesquiterpene lactones from Centaurea musimomum. Antiplasmodial and cytotoxic activities. Fitoterapia 76: 744-746. et al. 2005), we have taken interest in to study Centaurea sphaerocephala L. Previous studies on this species and/or its subspecies led to the isolation of flavonoid aglycones (Bentamene et al. 2008), flavonoid glucosides (Bentamene et al. 2010BENTAMENE A, BOUCHEHAM R, BAZ M, BENAYACHE S, CRECHE J and BENAYACHE F. 2010. Flavonoid glucosides from Centaurea sphaerocephala. Chem Nat Compd 46: 452-453.), sesquiterpene lactones (BrunoBRUNO M, FAZIO C, PASSANANTI S, PATERNOSTRO MP, DÍAZ JG and HERZ W. 1994. Sesquiterpene lactones from Centaurea sphaerocephala ssp. sphaerocephala. Phytochemistry 35: 1371-1372. et al. 1994, GeppertGEPPERT B, DROŻDŻ B, KIEŁCZEWSKI M and HOLUB M. 1983. Sesquiterpene lactones. XXIII. Isolation of sesquiterpene lactones from Centaurea L. species. Acta Soc Bot Pol 52: 23-34. et al. 1983), lignans, sesquilignans and a dithienylacetylene (BastosBASTOS MM, KIJJOA A, CARDOSO JM, GUTIÉRREZ AB and HERZ W. 1990. Lignans and other constituents of Centaurea sphaerocephala ssp. Polyacantha. Planta Med 56: 403-405. et al. 1990). The composition of the essential oil of this species was also reported (SenatoreSENATORE F, LANDOLFI S, CELIK S and BRUNO M. 2006. Volatile components of Centaurea calcitrapa L. and Centaurea sphaerocephala L. ssp. sphaerocephala, two Asteraceae growing wild in Sicily. Flavour Frag J 21: 282-285. et al. 2006).
Our previous study (LahnecheLAHNECHE AM, BOUCHEHAM R, BOUBEKRI N, BENSACI S, BICHA S, BENTAMENNE A, BENAYACHE F, BENAYACHE S and ZAMA D. 2017. Sodium Valproate-Induced Hepatic Dysfunction in Albino Rats and Protective Role of n-Butanol Extract of Centaurea sphaerocephala L. Intern J Pharm Phytochem Res 9(10):1335-1343. et al. 2017) showed that n-butanol (n-BuOH) extract of C. sphaerocephala exhibited significant protection against VPA-induced toxicity by its ability to ameliorate the lipid peroxidation and free radical (DPPH·) scavenging activity, which enhanced the levels of an antioxidant defense system. This effect could be attributed to its antioxidant properties (BekhoucheBEKHOUCHE K, OZEN T, BOUSSAHA S, KOLDAS S, YENIGUN S, LASSED S, DEMIRTAS I, BENAYACHE F, BENAYACHE S and ZAMA D. 2018. Anti-oxidant, DNA-damage protection and anti-cancer properties of n-butanol extract of the endemic Perralderia coronopifolia. Bangladesh J Pharmac 13: 82-89. et al. 2018).
Therefore, to our knowledge and as a part of our ongoing research program on beneficial health effects of plants and herbs (BoussahaBOUSSAHA S, BEKHOUCHE K, BOUDJERDA A, LEON F, KOLDAS S, YAGLIOGLU AS, DEMIRTAS I, BROUARD I, MARCHIONI E and ZAMA D. 2015. Chemical Constituents, in vitro Antioxidant and Antiproliferative Activities of Perralderia coronopifolia Coss. subsp. eu-coronopifolia M. var. typica M. extract. Rec Nat Prod 9: 312-322. et al. 2015, LassedLASSED S, DEUS CM, DJEBBARI R, ZAMA D, OLIVEIRA PJ, RIZVANOV AA, DAHDOUH A, BENAYACHE F and BENAYACHE S. 2017. Protective effect of green tea (Camellia sinensis (L.) Kuntze) against prostate cancer: from in vitro data to Algerian patients. Evid-Based Compl Alt 2017. et al. 2017), we investigate in the present study, the free radical scavenging activity using DPPH· test, phenol and flavonoid contents in the ethyl acetate (EtOAc) and the n-BuOH extracts of C. sphaerocephala. n-BuOH extract was also assessed for its inhibition of lipid peroxidation, antiproliferative activity, and oxidative DNA damage.
MATERIALS AND METHODS
PLANT MATERIAL AND EXTRACTION
Aerial parts of C. sphaerocephala were collected from the area of El Kala, Algeria (21 m, 36° 53’ 44” N, 8° 26’ 35” E) in May 2012 and authenticated on the basis of Quézel and Santa (1963) by Professor M. Kaabache, specialist in the identification of Algerian Centaurea species (Ferhat Abbas University, Setif 1, Algeria). A voucher specimen (CSA0512-EK-ALG-65) was deposited in the Herbarium of the VARENBIOMOL research unit, Frères Mentouri University, Constantine 1.
The leaves and flowers (2 kg) of this plant were macerated for 24 h, three times with methanol-water (70:30, v/v) at room temperature. After filtration, the clear filtrate was concentrated at 35 °C under vacuum, the remaining solution (400 mL) was dissolved in distilled H2O (800 mL) under magnetic stirring and kept at 4 °C overnight to precipitate a maximum amount of chlorophylls. After filtrating on a filter paper of Watman no: 1, the resulting solution was extracted with chloroform, EtOAc and n-BuOH, respectively. The solutions were dried with sodium sulfate (Na2SO4), filtered using normal filter paper and concentrated at 35 °C in a low vacuum to obtain the following extracts: CHCl3 (5.00 g), EtOAc (4.94 g) and n-BuOH (34.00 g).
DETERMINATION OF PHENOLIC CONTENTS
The total phenolic contents of the n-BuOH and the EtOAc extracts were estimated using Folin-Ciocalteu assay as given by SingletonSINGLETON VL, ORTHOFER R and LAMUELA-RAVENTÓS RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol: Elsevier, p. 152-178. (Singleton et al. 1999). Folin-Ciocalteu reagent was mixed with the extract or gallic acid (GA) solutions. After 3 min, 3 mL of Na2CO3 (20%) was added and then the mixture was incubated at room temperature for 2 hours. The absorbance was measured at 765 nm. All tests were performed in triplicate. GA was used as a standard phenolic compound. The phenolic content was calculated and expressed as a µg GA equivalent per mg of extract (GAE µg/mg extract).
TOTAL FLAVONOIDS
The determination of the total flavonoids in both extracts was estimated by the aluminum chloride colorimetric assay (WangWANG H, GAO XD, ZHOU GC, CAI L and YAO WB. 2008. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem 106: 888-895. et al. 2008). A volume of AlCl3 (2%) methanol solution was added to the same volume of sample. After incubation time for one hour at room temperature, the absorbance was then measured at 420 nm. Quercetin was used as a standard compound. The content of total flavonoids was calculated and expressed as a µg quercetin equivalent (QE) per mg of extracts (µg QE/mg extract).
RADICAL SCAVENGING ACTIVITY (DPPH· METHOD)
The activity of the n-BuOH and EtOAc extracts was performed using the DPPH method as described by BracaBRACA A, DE TOMMASI N, DI BARI L, PIZZA C, POLITI M and MORELLI I. 2001. Antioxidant principles from Bauhinia terapotensis. J Nat Prod 64: 892-895. (Braca et al. 2001). This method is based on the principal scavenging of the DPPH to evaluate radical scavenging activity. Briefly, different concentrations of each extract were added to 3 mL of 0.004% of the methanolic solution of DPPH·. The reaction mixture was kept in the dark for a period of 30 min. The absorbance was then monitored at 517 nm. Ascorbic acid was used as a standard. The inhibitory percentage (I, %) of the DPPH· was calculated according to the following equation:
where Acontrol is the absorbance of DPPH· solution, Asample is the absorbance of the sample. The IC50 of each extract was calculated from the graph plotted of inhibition percentage against the different concentrations of n-BuOH and EtOAc extracts. This test was carried out in triplicate at each concentration.
INHIBITION OF LIPID PEROXIDATION
The modified protocol reported by CaoCAO Y and IKEDA I. 2009. Antioxidant activity and antitumor activity (in vitro) of xyloglucan selenious ester and surfated xyloglucan. Int J Biol Macromol 45: 231-235. and Ikeda (Cao and Ikeda 2009) was performed to evaluate the capacity of the n-BuOH extract to inhibit lipid peroxidation using egg vitellose. As described in our previously published work (LassedLASSED S, AMRANI A, ALTUN M, ZAMA D, DEMIRTAS I, BENAYACHE F and BENAYACHE S. 2015. In vitro antioxidant, inhibition of oxidative DNA damage and antiproliferative activities of ethanolic green tea (Camellia sinensis) extract. Int J Pharm Sci Rev Res 35: 36-42. et al. 2015), the assay uses the egg vitellose as a source of lipids. 50 µL of FeSO4 (0.07 M) was mixed with 10% egg vitellose homogenate to induce lipid peroxidation, and then incubated at 37 °C with different concentrations of the extract or ascorbic acid. After a period of incubation for 30 min, 1.5 mL of 2-thiobarbituric acid (TBA, 1%) and 1 mL of trichloroacetic acid (TCA, 20%) were added. The samples were mixed and then incubated at 95 °C for 15 min. After centrifugation, the absorbance of the upper solution was monitored at 532 nm.
The percentage inhibition (I, %) of lipid peroxidation level was calculated using the following equation:
where Acontrol is the absorbance of control and Asample is the absorbance of the standard or sample.
THE INHIBITION CAPACITY OF DNA DAMAGE INDUCED BY OXIDATIVE STRESS
The protective capacity of n-BuOH extract of C. sphaerocephala against DNA damage was tested on 46966 plasmid DNA (extracted from E. coli). Plasmid DNA was oxidized with H2O2 via UV radiation in the presence or absence of the n-BuOH extract and performing agarose gel electrophoresis with the irradiated DNA (Russo et al. 2001). In two polyethylene microcentrifuge tubes, 1 µL aliquots of 46966 plasmid (200 µg/mL) were added, followed by 50 µg of n-BuOH extract in one of the two tubes. The other tube was irradiated control (CR). Then, 4 µL of 3% H2O2 was added to each tube. Next, they were placed on the surface of a UV transilluminator (300 nm) during 10 min, directly. In another tube, 1 µL aliquot of 46966 plasmid DNA was placed and served as a non-irradiated control (CO). All samples were run on 1% agarose gel and then photographed using a Lourmat gel imaging system (Vilber) (Lassed et al. 2015).
EVALUATION OF THE ANTICANCER ACTIVITY OF EXTRACTS USING XCELLigence SYSTEM
Cell Culture and XCELLigence
The Real-Time Cell Analyzer (XCELLigence, RTCA) (ACEABIO, USA) was used for the antiproliferative effect of the n-BuOH extract on human cervical cancer (HeLa) cells. The system measured impedance differences to derive cell index values at time points and it may be set by the operator. The cell activity depended on the cell index values and allowed cell behavior in a label-free environment and produced a real-time profile of the cells (Koldaş et al. 2015, CeyhanCEYHAN G, KOSE M, TUMER M and DEMIRTAS I. 2015. Anticancer, photoluminescence and electrochemical properties of structurally characterized two imine derivatives. Spectrochim Acta A 149: 731-743. et al. 2015). HeLa cells were cultured and supplemented with 10% (v/v) heat-inactivated fetal bovine serum using 2% penicillin-streptomycin at 37 °C in a humidified 5% CO2 atmosphere. HeLa cells in the culture flask were detached from the bottom of the flask by 10 mL of trypsin-EDTA mixture. The culture medium was added to this cell suspension at the same volume and then mixed gently. The suspension was centrifuged at 600 rpm at Falcon tubes, then removing the upper solution; 5 mL of medium was transferred carefully to the tube and carefully mixed. The concentrations of cell suspensions were determined by CEDEX HIRES Cell Counter using Trypan Blue (Boussaha et al. 2015, KoldasKOLDAS S, DEMIRTAS I, OZEN T, DEMIRCI MA and BEHCET L. 2015. Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a plant of traditional usage. J Sci Food Agr 95: 786-798. et al. 2015, Ceyhan et al. 2015).
Preparation of E-Plate 96 and Treatment
The culture medium (50 µL) was placed to each well and left in the hood for 15 min. The sterile incubator to allow the electrodes to equilibrate with the culture medium was inserted into the RTCA-SP station. The background measurement was performed for 1 minute to see the condition of the plate (Step 1). The plate was ejected from the station and 100 µL of cell suspension (2.5x104 cells/100 μL) was added to each well. Three blank wells were left to determine the culture medium. The plate was also left for another 30 min for the cells to adhere to the bottom and the measurement was performed for 80 min (step 2). At this stage, the cells began to attach to the electrodes on the plate. The cell index value increased as the cells attached to the surface. At the end of the step 2, the different extract solutions (10, 20 and 50 μL equivalent to 50, 100 and 250 μg/mL concentrations, respectively) were added to the wells and the final volumes were completed to 200 μL. Lastly, the plate was placed into the station and a measurement lasting 48 h was started (Step 3). During step 3, the status of the cells was recorded every 10 min with XCELLigence RTCA instrument. This measurement was made in triplicate (Boussaha et al. 2015, Koldas et al. 2015, Ceyhan et al. 2015). In apparel, cis-platin as a standard control at the doses of 250, 100, 50 and 10 μg/mL was used by following the same steps.
STATISTICAL ANALYSIS
Data are expressed as a mean ± standard deviation (SD). The statistical interferences were based on student’s test for mean values and compare to standard. Differences were highly significant at p < 0.01 and significant at p < 0.05.
RESULTS AND DISCUSSION
TOTAL PHENOLIC AND FLAVONOID CONTENTS OF THE EtOAc AND THE n-BuOH EXTRACTS
The total phenolic content of C. sphaerocephala was higher in the EtOAc extract: 357±11.32 μg GAE/mg extract compared to the n-BuOH extract: 202.5±1.5 μg GAE/mg extract. However, the amounts of flavonoids in the EtOAc and the n-BuOH extracts were 283.2±4.17 and 273.8±5.38 µg QE/mg extract, respectively. The high amount of total phenol content in both extracts justified their high antioxidant activities. Therefore, the antioxidant activity of plant extracts may result from phenolic compounds. Similar results were found in other studies using different plants: Caesalpinia bonducella seeds (ShuklaSHUKLA S, MEHTA A, JOHN J, SINGH S, MEHTA P and VYAS SP. 2009. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol 47: 1848-1851. et al. 2009), Chromolaema odorata leaves (RaoRAO KS, CHAUDHURY PK and PRADHAN A. 2010. Evaluation of anti-oxidant activities and total phenolic content of Chromolaena odorata. Food Chem Toxicol 48: 729-732. et al. 2010), biorefining of Bergenia crassifolia root and leaves (KraujalienėKRAUJALIENĖ V, PUKALSKAS A, KRAUJALIS P and VENSKUTONIS PR. 2016. Biorefining of Bergenia crassifolia L. roots and leaves by high pressure extraction methods and evaluation of antioxidant properties and main phytochemicals in extracts and plant material. Ind Crop Prod 89: 390-398. et al. 2016); different populations of lavandin (BajalanBAJALAN I, MOHAMMADI M, ALAEI M and PIRBALOUTI AG. 2016. Total phenolic and flavonoid contents and antioxidant activity of extracts from different populations of lavandin. Ind Crop Prod 87: 255-260. et al. 2016) and some plants from semiarid Mexican region (Wong-PazWONG-PAZ JE, CONTRERAS-ESQUIVEL JC, RODRÍGUEZ-HERRERA R, CARRILLO-INUNGARAY ML, LÓPEZ LI, NEVÁREZ-MOORILLÓN GV and AGUILAR CN. 2015. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac J Trop Med 8: 104-111. et al. 2015). A lignin, arctigenin, and a number of flavonoids, 3-O-glucosyl-isorhamnetin, rutin, 6-C-glucosyl-luteolin, isoquercitrin, trifolin, isoscoparin and isovitexin were previously isolated from aerial parts of C. macrocephala (SarkerSARKER SD, SAVCHENKO T, WHITING P, ŠIK V and DINAN LN. 1997. Moschamine, cis-moschamine, moschamindole and moschamindolol: four novel indole alkaloids from Centaurea moschata. Nat Prod Lett 9: 189-199. et al. 1997, RibeiroRIBEIRO N, NAHAR L, KUMARASAMY Y, MIR-BABAYEV N and SARKER S. 2002. Flavonoid C-glucosides and a lignan from Centaurea macrocephala (Compositae). Biochem Syst Ecol 30: 1097-1100. et al. 2002). The isolation, identification, toxicity and antioxidant activities of the isolated lignans such as arctiin, matairesinoside, matairesinol and lappaol A from seeds of C. macrocephala were also reported (ShoebSHOEB M, RAHMAN MM, NAHAR L, DELAZAR A, JASPARS M and MACMANUS SM. 2004. Bioactive lignans from the seeds of Centaurea macrocephala. Daru 12: 87-93. et al. 2004).
FREE RADICAL SCAVENGING ACTIVITY OF THE EtOAc AND THE n-BuOH EXTRACTS
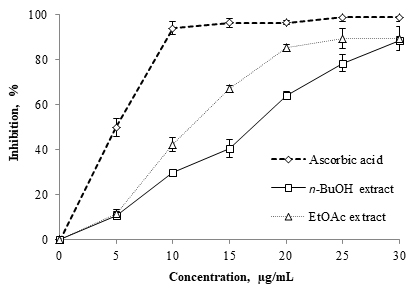
DPPH· has been used to determine free radical scavenging capacity of plant extracts (ErenlerERENLER R, YILMAZ S, AKSIT H, SEN O, GENC N, ELMASTAS M and DEMIRTAS I. 2014. Antioxidant activities of chemical constituents isolated from Echinops orientalis Trauv. Rec Nat Prod 8: 32. et al. 2014). As showed in Fig. 1, the scavenging ability of the EtOAc and the n-BuOH extracts was concentration-dependent (Erenler et al. 2014). The IC50 values were calculated as the concentration of the sample that caused the inhibition of 50% of free radical (DPPH·). A lower IC50 value indicated a higher scavenging activity. In this study, both extracts proved to be effective scavengers of DPPH·. The IC50 values of ascorbic acid, EtOAc and n-BuOH extracts were 5.18±0.12 μg/mL, 11.59±0.04 μg/mL and 16.67±0.11 μg/mL, respectively.
Free radical scavenging capacity of EtOAc and n-BuOH extracts of C. sphaerocephala using DPPH.
LIPID PEROXIDATION INHIBITORY ACTIVITY OF THE n-BuOH EXTRACT
In the current work, we investigated the potential of the n-BuOH extract of C. sphaerocephala in inhibiting the lipid peroxidation induced by the FeSO4 system, using egg vitellose homogenate. As shown in Fig. 2, the IC50 values of the inhibitory effect of the extract and ascorbic acid were 340.94±7.49 μg/mL and 20.62±0.93 μg/mL, respectively. The percentage inhibition of lipid peroxidation of the n-BuOH extract of C. sphaerocephala (100 and 200 µg/mL) was found to be 17.36% and 37.50%. While the ratio at the same concentrations for ascorbic acid was found to be 86.95% and 97.50% ,respectively. The n-BuOH extract decreased lipid peroxidation through its antioxidant and anti-radical power. In biological systems, lipid peroxidation generates a number of cytotoxic products, such as MDA, a marker of the oxidative stress in tissues injury (ZamaZAMA D, MERAIHI Z, TEBIBEL S, BENAYSSA W, BENAYACHE F, BENAYACHE S and VLIETINCK A. 2007. Chlorpyrifos-induced oxidative stress and tissue damage in the liver, kidney, brain and fetus in pregnant rats: The protective role of the butanolic extract of Paronychia argentea L. Indian J Pharm 39: 145-150. et al. 2007).
Inhibition of lipid peroxidation induced by n-BuOH extract of C. sphaerocephala and ascorbic acid.
DNA DAMAGE INHIBITION EFFICIENCY OF THE n-BuOH EXTRACT
Several studies provide evidence that DNA is susceptible to oxidative damage induced by free radicals in several diseases such as cancer. In order to investigate the protective effect of the n-BuOH extract of C. sphaerocephala, the UV-photolysis of H2O2 test was performed (Fig. 3). The untreated nonirradiated DNA (CO) generated one band corresponding to supercoiled circular DNA which the native form is normally found in vivo. While both the irradiated control and the test sample (containing 50 µg of the n-BuOH extract) showed two new forms of DNA: the relaxed circular and the linear forms. These forms naturally occur when the DNA is nicked and damaged. In our case, the extract did not show any ability in protecting DNA against UV photolysis of H2O2 induced damage and did not keep DNA in its native supercoiled form (OzakiOZAKI T, NAKAMURA M and SHIMOZATO O. 2015. Novel implications of DNA damage response in drug resistance of malignant cancers obtained from the functional interaction between p53 family and RUNX2. Biomol 5: 2854-2876. et al. 2015, ValkoVALKO M, MORRIS H, MAZUR M, RAPTA P and BILTON RF. 2001. Oxygen free radical generating mechanisms in the colon: do the semiquinones of vitamin K play a role in the aetiology of colon cancer? BBA-Gen 1527: 161-166. et al. 2001).
Effect of n-BuOH extract of C. sphaerocephala on the safety of 46966 plasmid DNA against oxidative damage caused by UV-photolysed H2O2.Co: untreated non-irradiated DNA.CR: untreated UV-irradiated DNA Sample: DNA and UV-irradiated treated with n-BuOH extract. Form I: supercoiled plasmid DNA.Form II: open circular double stranded DNA. Form III: linear DNA
THE ANTIPROLIFERATIVE ACTIVITY OF THE n-BuOH EXTRACT
HeLa (human cervix carcinoma) cells were the first human cell line established in culture (LuceyLUCEY BP, NELSON-REES WA and HUTCHINS GM. 2009. Henrietta Lacks, HeLa cells, and cell culture contamination. Arch Path Lab Med 133: 1463-1467. et al. 2009) and meanwhile has become the most widely used human cell line in biological research (ManosroiMANOSROI J, BOONPISUTTINANT K, MANOSROI W and MANOSROI A. 2012. Anti-proliferative activities on HeLa cancer cell line of Thai medicinal plant recipes selected from MANOSROI II database. J Ethnopharmacol 142: 422-431. et al. 2012). The antiproliferative activity of the n-BuOH extract against HeLa cells was tested using XCELLigence RTCA software (Koldaş et al. 2015). In this study, antiproliferative effects of the n-BuOH extract obtained from C. sphaerocephala L. were examined on HeLa cell lines. The cis-platin as metal-based drug candidate molecules was proved to have antiproliferative activity. The last fifty years, many metal complexes such as cis-platin were carried out in vitro and in vivo biological assays. Therefore, the cis-platin was used as a positive control in this study. As represented in Figs. 4 and 5, in the first 80 minutes, cells not treated with extract or positive control (cis-platin) were observed to have normal growth, but the medium wells (green line) did not have any increase. After step 2, the extract or the positive control was added to the cell culture media of each well of E-plate 96 at different concentrations.
In vitro antiproliferative activity of n-BuOH extract of C. sphaerocephala against HeLa cell lines (2.5x104 cell/well). Each substance was tested twice in triplicates using RTCA xCELLigence instrument. Different concentrations of n-BuOH extract were applied to the cells represented by different color (
 250 μg/mL
250 μg/mL  100 μg/mL
100 μg/mL  50 μg/mL
50 μg/mL  Control
Control  Medium).
Medium).
In vitro antiproliferative activities of cis-platin at different concentrations indicated with colors (
 250 μg/mL
250 μg/mL  100 μg/mL
100 μg/mL  50 μg/mL
50 μg/mL  10 μg/mL
10 μg/mL  Control
Control  Medium).
Medium).
Antiproliferative activity of the n-BuOH extract at 100 and 50 μg/mL concentrations showed lower activity than 250 μg/mL. As shown in Fig. 4, the lowest activity (turquoise line) was observed at the lowest concentration of 50 μg/mL. The low concentration decreased antiproliferative activity stepwise after the first 10 h and cell index (CI) increased slowly during the period. However, CI values of low concentration were lower than control value (red line). The antiproliferative effect of 100 μg/mL concentration (pink line) was not as high as the highest dose but more efficient than a lower dose. The extract showed an interesting effect against HeLa cells especially between the 30 to 50 h. The best effects observed from n-BuOH extract at the higher concentration of 250 μg/mL compared to the positive and the negative controls and literature (Oke-AltuntasOKE-ALTUNTAS F, DEMIRTAS I, TUFEKCI AR, KOLDAS S, GUL F, BEHCET L and GECIBESLER HI. 2016. Inhibitory effects of the active components isolated from Satureja Boissieri Hausskn. Ex Boiss. On human cervical cancer cell line. J Food Biochem 40: 499-506. et al. 2016, YaglıogluYAGLıOGLU AS, AKDULUM B, ERENLER R, DEMIRTAS I, TELCI I and TEKIN S. 2013. Antiproliferative activity of pentadeca-(8E, 13Z) dien-11-yn-2-one and (E)-1, 8-pentadecadiene from Echinacea pallida (Nutt.) Nutt. roots. Med Chem Res 22: 2946-2953. et al. 2013).
These results showed that the extract exhibited different effect against HeLa cell line at different concentrations. Thus, the antiproliferative activity of the n-BuOH extract increased in a dose-depended manner from 50 to 250 μg/mL. It may be suggested that the antiproliferative properties of the n-BuOH extract of C. sphaerocephala could be attributed to the presence of phenolic compounds, especially flavonoids reported in previous study (Bruno et al. 1994). This class of natural compounds plays an important role in cancer prevention and treatment (Samet et al. 2014, Koldaş et al. 2015, AbayABAY G, ALTUN M, KOLDAS S, RIZA TUFEKCI A and DEMIRTAS I. 2015. Determination of antiproliferative activities of volatile contents and HPLC profiles of Dicranum scoparium (Dicranaceae, Bryophyta). Comb Chem High T Scr 18: 453-463. et al. 2015). Many studies reported that flavonoids have protective effects against some diseases as cancer. Flavonoids as isocoreopsin, butrin and isobutrin have an essential role in the chemoprevention of colon cancer (TeohTEOH PL, CHENG AYF, LIAU M, LEM FF, KALING GP, CHUA FN and CHEONG BE. 2017. Chemical composition and cytotoxic properties of Clinacanthus nutans root extracts. Pharm Biol 55: 394-401. et al. 2017). Plant polyphenols have drawn increasing attention due to their protective effects against several oxidative stress associated diseases such as cancer. Natural phenolics have been found to mediate all stages of cancer development (SubramaniyanSUBRAMANIYAN B, POLACHI N and MATHAN G. 2016. Isocoreopsin: An active constituent of n-butanol extract of Butea monosperma flowers against colorectal cancer (CRC). J Pharm Anal 6: 318-325. et al. 2016, YildizYILDIZ I, SEN O, ERENLER R, DEMIRTAS I and BEHCET L. 2017. Bioactivity–guided isolation of flavonoids from Cynanchum acutum L. subsp. sibiricum (willd.) Rech. f. and investigation of their antiproliferative activity. Nat Prod Res 31: 2629-2633. et al. 2017). In addition to their antioxidant activities, they inhibit cancer development via a number of cellular essential mechanisms (DaiDAI J and MUMPER RJ. 2010. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15: 7313-7352. and Mumper 2010).
These results confirmed other studies which reported that Centaurea species contain natural potent anticancer agents against a variety of human malignances including prostate, lung, colon, stomach, kidney, pancreas and mammary glands (YagliogluYAGLIOGLU AS, DEMIRTAS I and GOREN N. 2014. Bioactivity-guided isolation of antiproliferative compounds from Centaurea carduiformis DC. Phytochem Lett 8: 213-219. et al. 2014). This beneficial effect has been attributed to the presence of high amounts of polyphenols, which are potent antioxidants especially sesquiterpene lactones (ErenlerERENLER R, SEN O, SAHIN YAGLIOGLU A and DEMIRTAS I. 2016. Bioactivity-guided isolation of antiproliferative sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Comb Chem High T Scr 19: 66-72. et al. 2016, DemirtasDEMIRTAS I, ERENLER R, ELMASTAS M and GOKTASOGLU A. 2013. Studies on the antioxidant potential of flavones of Allium vineale isolated from its water-soluble fraction. Food Chem 136: 34-40. et al. 2013).
CONCLUSION
In the present study, both extracts of C. sphaerocephala showed strong antioxidant activity on scavenging of DPPH. Their high antioxidant property might be due to the presence of polyphenols and flavonoids. The n-BuOH extract inhibited lipid peroxidation. In addition, the n-BuOH extract showed antiproliferative activity against HeLa cell lines. It can be concluded that the n-BuOH extract of C. sphaerocephala L. has antiproliferative components. The strong antiproliferative activity at the high concentration of this extract suggests that this plant may be a promising source of natural antioxidant and functional plant material. These findings need extensive studies on the isolation and the determination of chemical profiles and the mechanism of antiproliferative and antioxidant activities.
ACKNOWLEGMENTS
The authors thank the Algerian Ministry of Higher Education and Scientific Research (MESRS) for financial support. The authors also thank Professor M. Kaabeche for the identification of the plant material and Professor S. Khennouf for improving the English quality of this manuscript.
REFERENCES
- ABAY G, ALTUN M, KOLDAS S, RIZA TUFEKCI A and DEMIRTAS I. 2015. Determination of antiproliferative activities of volatile contents and HPLC profiles of Dicranum scoparium (Dicranaceae, Bryophyta). Comb Chem High T Scr 18: 453-463.
- ACLINOU P, BOUKERB A, BOUQUANT J, MASSIOT G and MEN-OLIVER L. 1982. Plantes des Aures: constituants des racines de Centaurea incana (Composes). Plant Med Phytother 16: 303-309.
- AÏCHA MHO. 2017. Cholinestérases et toxicité d’extraits de quelques plantes acridicides ou acridifuges chez Schistocerca gregaria (Forskål, 1775). Thèse de Doctorat. Universite Kasdi Merbah Ouargla.
- BAJALAN I, MOHAMMADI M, ALAEI M and PIRBALOUTI AG. 2016. Total phenolic and flavonoid contents and antioxidant activity of extracts from different populations of lavandin. Ind Crop Prod 87: 255-260.
- BASTOS MM, KIJJOA A, CARDOSO JM, GUTIÉRREZ AB and HERZ W. 1990. Lignans and other constituents of Centaurea sphaerocephala ssp. Polyacantha. Planta Med 56: 403-405.
- BAYTOP T. 1999. Türkiye’de Bitkiler ile Tedavi (Geçmiste ve Bugün) İlaveli Ikinci Baskı. Nobel Tıp Kitabevleri, (No:3255), Istanbul (in Turkish), p. 316.
- BEKHOUCHE K, OZEN T, BOUSSAHA S, KOLDAS S, YENIGUN S, LASSED S, DEMIRTAS I, BENAYACHE F, BENAYACHE S and ZAMA D. 2018. Anti-oxidant, DNA-damage protection and anti-cancer properties of n-butanol extract of the endemic Perralderia coronopifolia. Bangladesh J Pharmac 13: 82-89.
- BENTAMENE A, BAZ M, BOUCHEHAM R, BENAYACHE S, CRECHE J and BENAYACHE F. 2008. Flavonoid aglycones from Centaurea sphaerocephala. Chem Nat Compd 2: 234-235.
- BENTAMENE A, BOUCHEHAM R, BAZ M, BENAYACHE S, CRECHE J and BENAYACHE F. 2010. Flavonoid glucosides from Centaurea sphaerocephala. Chem Nat Compd 46: 452-453.
- BICHA S, BENTAMENE A, BENAISSA O, BENAYACHE S, GARCIA V, LEON F, BROUARD I, BERMEJO J and BENAYACHE F. 2011. Flavonoid aglycones from Centaurea maroccana. Chem Nat Compd 47: 105-106.
- BICHA S, CHALARD P, HAMMOUD L, LEÓN F, BROUARD I, GARCIA VP, LOBSTEIN A, BENTAMENE A, BENAYACHE S and BERMEJO J. 2013. Maroccanin: A New g-lactone and Other Constituents from Centaurea maroccana Ball. (Asteraceae). Rec Nat Prod 7: 114-118.
- BORA KS and SHARMA A. 2011. Evaluation of antioxidant and free-radical scavenging potential of Artemisia absinthium. Pharm Biol 49: 1216-1223.
- BOUSSAHA S, BEKHOUCHE K, BOUDJERDA A, LEON F, KOLDAS S, YAGLIOGLU AS, DEMIRTAS I, BROUARD I, MARCHIONI E and ZAMA D. 2015. Chemical Constituents, in vitro Antioxidant and Antiproliferative Activities of Perralderia coronopifolia Coss. subsp. eu-coronopifolia M. var. typica M. extract. Rec Nat Prod 9: 312-322.
- BRACA A, DE TOMMASI N, DI BARI L, PIZZA C, POLITI M and MORELLI I. 2001. Antioxidant principles from Bauhinia terapotensis. J Nat Prod 64: 892-895.
- BRUNO M, FAZIO C, PASSANANTI S, PATERNOSTRO MP, DÍAZ JG and HERZ W. 1994. Sesquiterpene lactones from Centaurea sphaerocephala ssp. sphaerocephala. Phytochemistry 35: 1371-1372.
- CAO Y and IKEDA I. 2009. Antioxidant activity and antitumor activity (in vitro) of xyloglucan selenious ester and surfated xyloglucan. Int J Biol Macromol 45: 231-235.
- CEYHAN G, KOSE M, TUMER M and DEMIRTAS I. 2015. Anticancer, photoluminescence and electrochemical properties of structurally characterized two imine derivatives. Spectrochim Acta A 149: 731-743.
- ĆIRIĆ A, KARIOTI A, GLAMOČLIJA J, SOKOVIĆ M and SKALTSA H. 2011. Antimicrobial activity of secondary metabolites isolated from Centaurea spruneri Boiss. & Heldr. J Serb Chem Soc 76: 27-34.
- CRAGG GM and NEWMAN DJ. 2013. Natural products: a continuing source of novel drug leads. BBA-GEN Subjects 1830: 3670-3695.
- DAI J and MUMPER RJ. 2010. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15: 7313-7352.
- DEMIRTAS I, ERENLER R, ELMASTAS M and GOKTASOGLU A. 2013. Studies on the antioxidant potential of flavones of Allium vineale isolated from its water-soluble fraction. Food Chem 136: 34-40.
- ERENLER R, SEN O, SAHIN YAGLIOGLU A and DEMIRTAS I. 2016. Bioactivity-guided isolation of antiproliferative sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Comb Chem High T Scr 19: 66-72.
- ERENLER R, YILMAZ S, AKSIT H, SEN O, GENC N, ELMASTAS M and DEMIRTAS I. 2014. Antioxidant activities of chemical constituents isolated from Echinops orientalis Trauv. Rec Nat Prod 8: 32.
- FAKHFAKH JAE and DAMAK M. 2007. Sesquineolignans from the flowers of Centaurea furfuracea Coss. et Dur.(Asteraceae). Nat Prod Res 21: 1037-1041.
- GEPPERT B, DROŻDŻ B, KIEŁCZEWSKI M and HOLUB M. 1983. Sesquiterpene lactones. XXIII. Isolation of sesquiterpene lactones from Centaurea L. species. Acta Soc Bot Pol 52: 23-34.
- KARIOTI A, SKALTSA H, LAZARI D, SOKOVIC M, GARCIA B and HARVALA C. 2002. Secondary metabolites from Centaurea deusta with antimicrobial activity. Z Naturforsch C 57: 75-80.
- KHODAIE L, BAMDAD S, DELAZAR A and NAZEMIYEH H. 2012. Antioxidant, total phenol and flavonoid contents of two pedicularis l. species from Eastern Azerbaijan, Iran. Bioimpacts: BI 2: 43-57.
- KOLDAS S, DEMIRTAS I, OZEN T, DEMIRCI MA and BEHCET L. 2015. Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a plant of traditional usage. J Sci Food Agr 95: 786-798.
- KOLLI EH, LEÓN F, BENAYACHE F, ESTÉVEZ S, QUINTANA J, ESTÉVEZ F, BROUARD I, BERMEJO J and BENAYACHE S. 2012. Cytotoxic sesquiterpene lactones and other constituents of Centaurea omphalotricha. J Braz Chem Soc 23: 977-983.
- KOUKOULITSA C, GEROMICHALOS GD and SKALTSA H. 2005. VolSurf analysis of pharmacokinetic properties for several antifungal sesquiterpene lactones isolated from Greek Centaurea sp. J Comput Aid Mol Des 19: 617-623.
- KOUKOULITSA E, SKALTSA H, KARIOTI A, DEMETZOS C and DIMAS K. 2002. Bioactive sesquiterpene lactones from Centaurea species and their cytotoxic/cytostatic activity against human cell lines in vitro. Planta Med 68: 649-652.
- KRAUJALIENĖ V, PUKALSKAS A, KRAUJALIS P and VENSKUTONIS PR. 2016. Biorefining of Bergenia crassifolia L. roots and leaves by high pressure extraction methods and evaluation of antioxidant properties and main phytochemicals in extracts and plant material. Ind Crop Prod 89: 390-398.
- LAHNECHE AM, BOUCHEHAM R, BOUBEKRI N, BENSACI S, BICHA S, BENTAMENNE A, BENAYACHE F, BENAYACHE S and ZAMA D. 2017. Sodium Valproate-Induced Hepatic Dysfunction in Albino Rats and Protective Role of n-Butanol Extract of Centaurea sphaerocephala L. Intern J Pharm Phytochem Res 9(10):1335-1343.
- LASSED S, AMRANI A, ALTUN M, ZAMA D, DEMIRTAS I, BENAYACHE F and BENAYACHE S. 2015. In vitro antioxidant, inhibition of oxidative DNA damage and antiproliferative activities of ethanolic green tea (Camellia sinensis) extract. Int J Pharm Sci Rev Res 35: 36-42.
- LASSED S, DEUS CM, DJEBBARI R, ZAMA D, OLIVEIRA PJ, RIZVANOV AA, DAHDOUH A, BENAYACHE F and BENAYACHE S. 2017. Protective effect of green tea (Camellia sinensis (L.) Kuntze) against prostate cancer: from in vitro data to Algerian patients. Evid-Based Compl Alt 2017.
- LUCEY BP, NELSON-REES WA and HUTCHINS GM. 2009. Henrietta Lacks, HeLa cells, and cell culture contamination. Arch Path Lab Med 133: 1463-1467.
- MANOSROI J, BOONPISUTTINANT K, MANOSROI W and MANOSROI A. 2012. Anti-proliferative activities on HeLa cancer cell line of Thai medicinal plant recipes selected from MANOSROI II database. J Ethnopharmacol 142: 422-431.
- MEDJROUBI K, BENAYACHE F and BERMEJO J. 2005. Sesquiterpene lactones from Centaurea musimomum. Antiplasmodial and cytotoxic activities. Fitoterapia 76: 744-746.
- OKE-ALTUNTAS F, DEMIRTAS I, TUFEKCI AR, KOLDAS S, GUL F, BEHCET L and GECIBESLER HI. 2016. Inhibitory effects of the active components isolated from Satureja Boissieri Hausskn. Ex Boiss. On human cervical cancer cell line. J Food Biochem 40: 499-506.
- OZAKI T, NAKAMURA M and SHIMOZATO O. 2015. Novel implications of DNA damage response in drug resistance of malignant cancers obtained from the functional interaction between p53 family and RUNX2. Biomol 5: 2854-2876.
- OZENDA P. 1958. Flore du Sahara Septentrional et Central. Centre National de la Recherche Scientifique(CNRS), Paris, p. 450-454.
- QUÉZEL P and SANTA S. 1963. Nouvelle flore de l’Algérie et des régions désertiques méridionales: Centre National de La Recherche Scientifique (CNRS) Paris, Tome II., 1963, p 1032.
- RAO KS, CHAUDHURY PK and PRADHAN A. 2010. Evaluation of anti-oxidant activities and total phenolic content of Chromolaena odorata. Food Chem Toxicol 48: 729-732.
- REYHAN A, KÜPELİ E and ERGUN F. 2004. The biological activity of Centaurea L. species. Gazi U J Sci 17: 149-164.
- RIBEIRO N, NAHAR L, KUMARASAMY Y, MIR-BABAYEV N and SARKER S. 2002. Flavonoid C-glucosides and a lignan from Centaurea macrocephala (Compositae). Biochem Syst Ecol 30: 1097-1100.
- RUSSO A, IZZO A, CARDILE V, BORRELLI F and VANELLA A. 2001. Indian medicinal plants as antiradicals and DNA cleavage protectors. Phytomedicine 8: 125-132.
- SADEGHI Z, VALIZADEH J, SHERMEH OA and AKABERI M. 2015. Antioxidant activity and total phenolic content of Boerhavia elegans (choisy) grown in Baluchestan, Iran. Avicenna J Phytomed 5: 1-9.
- SAMET I, HAN J, JLAIEL L, SAYADI S and ISODA H. 2014. Olive (Olea europaea) leaf extract induces apoptosis and monocyte/macrophage differentiation in human chronic myelogenous leukemia K562 cells: insight into the underlying mechanism. Oxid Med Cell Longev 2014: 1-17.
- SARKER SD, SAVCHENKO T, WHITING P, ŠIK V and DINAN LN. 1997. Moschamine, cis-moschamine, moschamindole and moschamindolol: four novel indole alkaloids from Centaurea moschata. Nat Prod Lett 9: 189-199.
- SENATORE F, LANDOLFI S, CELIK S and BRUNO M. 2006. Volatile components of Centaurea calcitrapa L. and Centaurea sphaerocephala L. ssp. sphaerocephala, two Asteraceae growing wild in Sicily. Flavour Frag J 21: 282-285.
- SHOEB M, RAHMAN MM, NAHAR L, DELAZAR A, JASPARS M and MACMANUS SM. 2004. Bioactive lignans from the seeds of Centaurea macrocephala. Daru 12: 87-93.
- SHUKLA S, MEHTA A, JOHN J, SINGH S, MEHTA P and VYAS SP. 2009. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol 47: 1848-1851.
- SINGLETON VL, ORTHOFER R and LAMUELA-RAVENTÓS RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol: Elsevier, p. 152-178.
- SUBRAMANIYAN B, POLACHI N and MATHAN G. 2016. Isocoreopsin: An active constituent of n-butanol extract of Butea monosperma flowers against colorectal cancer (CRC). J Pharm Anal 6: 318-325.
- TEOH PL, CHENG AYF, LIAU M, LEM FF, KALING GP, CHUA FN and CHEONG BE. 2017. Chemical composition and cytotoxic properties of Clinacanthus nutans root extracts. Pharm Biol 55: 394-401.
- TING H-C, HSU Y-W, TSAI C-F, LU F-J, CHOU M-C and CHEN W-K. 2011. The in vitro and in vivo antioxidant properties of seabuckthorn (Hippophae rhamnoides L.) seed oil. Food Chem 125: 652-659.
- TUKOV FF, ANAND S, GADEPALLI RSV, GUNATILAKA AL, MATTHEWS JC and RIMOLDI JM. 2004. Inactivation of the cytotoxic activity of repin, a sesquiterpene lactone from Centaurea repens. Chem Res Toxicol 17: 1170-1176.
- VALKO M, MORRIS H, MAZUR M, RAPTA P and BILTON RF. 2001. Oxygen free radical generating mechanisms in the colon: do the semiquinones of vitamin K play a role in the aetiology of colon cancer? BBA-Gen 1527: 161-166.
- WANG H, GAO XD, ZHOU GC, CAI L and YAO WB. 2008. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem 106: 888-895.
- WONG-PAZ JE, CONTRERAS-ESQUIVEL JC, RODRÍGUEZ-HERRERA R, CARRILLO-INUNGARAY ML, LÓPEZ LI, NEVÁREZ-MOORILLÓN GV and AGUILAR CN. 2015. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac J Trop Med 8: 104-111.
- YAGLıOGLU AS, AKDULUM B, ERENLER R, DEMIRTAS I, TELCI I and TEKIN S. 2013. Antiproliferative activity of pentadeca-(8E, 13Z) dien-11-yn-2-one and (E)-1, 8-pentadecadiene from Echinacea pallida (Nutt.) Nutt. roots. Med Chem Res 22: 2946-2953.
- YAGLIOGLU AS, DEMIRTAS I and GOREN N. 2014. Bioactivity-guided isolation of antiproliferative compounds from Centaurea carduiformis DC. Phytochem Lett 8: 213-219.
- YILDIZ I, SEN O, ERENLER R, DEMIRTAS I and BEHCET L. 2017. Bioactivity–guided isolation of flavonoids from Cynanchum acutum L. subsp. sibiricum (willd.) Rech. f. and investigation of their antiproliferative activity. Nat Prod Res 31: 2629-2633.
- ZAMA D, MERAIHI Z, TEBIBEL S, BENAYSSA W, BENAYACHE F, BENAYACHE S and VLIETINCK A. 2007. Chlorpyrifos-induced oxidative stress and tissue damage in the liver, kidney, brain and fetus in pregnant rats: The protective role of the butanolic extract of Paronychia argentea L. Indian J Pharm 39: 145-150.
- ZATER H, HUET J, FONTAINE V, BENAYACHE S, STÉVIGNY C, DUEZ P and BENAYACHE F. 2016. Chemical constituents, cytotoxic, antifungal and antimicrobial properties of Centaurea diluta Ait. subsp. algeriensis (Coss. & Dur.) Maire. Asian Pac J Trop Med 9: 554-561.
Publication Dates
-
Publication in this collection
23 Sept 2019 -
Date of issue
2019
History
-
Received
10 May 2018 -
Accepted
20 Aug 2018