Abstract
A feasibility analysis of tertiary treatment for Organic Liquid Agricultural Waste is presented using filamentous algae belonging to the genus Cladophora sp. as an alternative to chemical tertiary treatment. The main advantages of tertiary treatments that use biological systems are the low cost investment and the minimal dependence on environmental variables. In this work we demonstrate that filamentous algae reduces the nutrient load of nitrate (circa 75%) and phosphate (circa 86%) from the organic waste effluents coming from dairy farms after nine days of culture, with the added advantage being that after the treatment period, algae removal can be achieved by simple procedures. Currently, the organic wastewater is discarded into fields and local streams. However, the algae can acquire value as a by-product since it has various uses as compost, cellulose, and biogas. A disadvantage of this system is that clean water must be used to achieve enough water transparency to allow algae growth. Even so, the nutrient reduction system of the organic effluents proposed is friendly to the ecosystem, compared to tertiary treatments that use chemicals to precipitate and collect nutrients such as nitrates and phosphates.
Keywords:
nutrient removal; phycoremediation; dairy wastewater
Resumo
Uma análise de viabilidade do tratamento terciário para Resíduos Agrícola Líquidos Orgânicos é apresentada usando algas filamentosas pertencentes ao gênero Cladophora sp. como alternativa ao tratamento químico terciário. Os tratamentos terciários que utilizam sistemas biológicos têm baixo custo de investimento e a dependência de variáveis ambientais é mínima. Neste trabalho, é demonstrado que essas algas filamentosas reduzem a carga nutricional de nitrato (circa 75%) e fosfato (circa 86%) dos efluentes de resíduos orgânicos provenientes de fazendas de leite em nove dias de cultura e tem a vantagem de que as algas podem ser facilmente coletadas posteriormente. Atualmente, as águas residuais orgânicas são descartadas nos campos e córregos locais. Posteriormente, as algas podem ser consideradas como matéria prima, uma vez que possuem várias utilidades como composto, celulose e biogás. Uma desvantagem desse sistema é que água limpa deve ser usada para obter transparência de água suficiente para permitir o crescimento de algas. Mesmo assim, o sistema de redução de nutrientes dos efluentes orgânicos propostos e amigável ao ecossistema, comparado aos tratamentos terciários que utilizam produtos químicos para precipitar e coletar nutrientes como nitratos e fosfatos.
Palavras-chave:
remoção de nutrientes; ficorremediação; águas residuais de laticínios
1. Introduction
Removal of nutrients from wastewater is very important to maintain the environmental health of aquatic systems. Heavy loads of nutrients to lacustrine systems may accelerate eutrophication and cause economic losses and sickness to animal and human communities (Aslan and Kapdan, 2006ASLAN, S. and KAPDAN, I.K., 2006. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecological Engineering, vol. 28, no. 1, pp. 64-70. http://dx.doi.org/10.1016/j.ecoleng.2006.04.003.
http://dx.doi.org/10.1016/j.ecoleng.2006...
). Chemical removal of nutrients such as nitrate and phosphate is costly and not environmentally friendly because it requires additional chemicals that generate secondary pollution (Shin and Lee, 1998SHIN, H.S. and LEE, S.M., 1998. Removal of Nutrients in Wastewater by using Magnesium Salts. Environmental Technology, vol. 19, no. 3, pp. 283-290. http://dx.doi.org/10.1080/09593331908616682.
http://dx.doi.org/10.1080/09593331908616...
). Therefore, a biological tertiary process would be most welcome since it allows the reuse of water and the utilization of the generated biomass.
Chile has 373 sewage treatment plants but only 8% of the plants have some type of tertiary treatment (Barañao and Tapia, 2004BARAÑAO, P.A. and TAPIA, L.A., 2004. Tratamiento de las Aguas Servidas: Situación en Chile. Ciencia & Trabajo, no. 13, pp. 111-117.). Plants with this type of treatment are divided into two groups: 65% use biological technologies to remove nitrogen and phosphorus, while 30% use chemicals to remove phosphorus (Barañao and Tapia, 2004BARAÑAO, P.A. and TAPIA, L.A., 2004. Tratamiento de las Aguas Servidas: Situación en Chile. Ciencia & Trabajo, no. 13, pp. 111-117.).
The green filamentous algae Cladophora sp. grows in almost all aquatic systems worldwide (Whitton, 1970WHITTON, B.A., 1970. Biology of Cladophora in freshwaters. Water Resources, vol. 4, pp. 457-476.; Dodds and Gudder, 1992DODDS, W.K. and GUDDER, D.A., 1992. The ecology of Cladophora.Journal of Phycology, vol. 28, no. 4, pp. 415-427. http://dx.doi.org/10.1111/j.0022-3646.1992.00415.x.
http://dx.doi.org/10.1111/j.0022-3646.19...
; Higgins et al., 2008HIGGINS, S.N., MALKIN, S.Y., TODD HOWELL, E., GUILDFORD, S.J., CAMPBELL, L., HIRIART-BAER, V. and HECKY, R.E., 2008. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. Journal of Phycology, vol. 44, no. 4, pp. 1-16. http://dx.doi.org/10.1111/j.1529-8817.2008.00538.x. PMid:27041601.
http://dx.doi.org/10.1111/j.1529-8817.20...
; Graham et al., 2018GRAHAM, L.E., GRAHAM, J.M., WILCOX, L.W. and COOK, M.E., 2018. Algae. 3rd ed. Madison, WI: LJLM Press. 616 p.). Light intensity determines the depth at which Cladophora can develop its biomass. In Lake Huron, Cladophora glomerata needed a minimum irradiance of 30 µmol photons*m-2*s-1 at the temperature range of 5-20 °C, to have a positive net photosynthesis (Graham et al., 1982GRAHAM, J.M., AUER, M.T., CANALE, R.P. and HOFFMANN, J.P., 1982. Ecological Studies and Mathematical Modeling of Cladophora in Lake Huron: 4. Photosynthesis and respiration as functions of light and temperature. Journal of Great Lakes Research, vol. 8, no. 1, pp. 100-111. http://dx.doi.org/10.1016/S0380-1330(82)71948-3.
http://dx.doi.org/10.1016/S0380-1330(82)...
). Cladophora sp. has been reported to grow at temperatures in the range of 13-35 °C (Graham et al., 1982GRAHAM, J.M., AUER, M.T., CANALE, R.P. and HOFFMANN, J.P., 1982. Ecological Studies and Mathematical Modeling of Cladophora in Lake Huron: 4. Photosynthesis and respiration as functions of light and temperature. Journal of Great Lakes Research, vol. 8, no. 1, pp. 100-111. http://dx.doi.org/10.1016/S0380-1330(82)71948-3.
http://dx.doi.org/10.1016/S0380-1330(82)...
; Lester et al.,1988LESTER, W.W., ADAMS, M.S. and FARMER, A.M., 1988. Effects of light and temperature on photosynthesis of the nuisance alga Cladophora glomerata (L.) Kütz. The New Phytologist, vol. 109, no. 1, pp. 53-58. http://dx.doi.org/10.1111/j.1469-8137.1988.tb00218.x.
http://dx.doi.org/10.1111/j.1469-8137.19...
; Higgins et al.,2005HIGGINS, S.N., TODD HOWELL, E., HECKY, R.E., GUILDFORD, S.J. and SMITH, R.E., 2005. The wall of green: The status of Cladophora glomerata on the northern shores of Lake Erie’s eastern basin, 1995-2002. Journal of Great Lakes Research, vol. 31, no. 4, pp. 547-563. http://dx.doi.org/10.1016/S0380-1330(05)70283-5.
http://dx.doi.org/10.1016/S0380-1330(05)...
) and at the optimal light intensity of 200-300 µmol photons*m-2*s-1 (Graham et al., 1982GRAHAM, J.M., AUER, M.T., CANALE, R.P. and HOFFMANN, J.P., 1982. Ecological Studies and Mathematical Modeling of Cladophora in Lake Huron: 4. Photosynthesis and respiration as functions of light and temperature. Journal of Great Lakes Research, vol. 8, no. 1, pp. 100-111. http://dx.doi.org/10.1016/S0380-1330(82)71948-3.
http://dx.doi.org/10.1016/S0380-1330(82)...
; Canale et al., 1982CANALE, R.P., AUER, M.T. and GRAHAM, J.M., 1982. Ecological studies and mathematical modeling of Cladophora in Lake Huron: 6. Seasonal and spatial Variation in Growth Kinetics. Journal of Great Lakes Research, vol. 8, no. 1, pp. 126-133. http://dx.doi.org/10.1016/S0380-1330(82)71950-1.
http://dx.doi.org/10.1016/S0380-1330(82)...
). Such a broad tolerance to irradiance and temperature has contributed to its invasive behavior in some habitats (Zulkifly et al., 2013ZULKIFLY, S.B., GRAHAM, J., YOUNG, E.B., MAYER, R.J., PIOTROWSKI, M.J., SMITH, I. and GRAHAM, L.E., 2013. The genus Cladophora Kützing (Ulvophyceae) as a globally distributed ecological engineer. Journal of Phycology, vol. 49, no. 1, pp. 1-17. http://dx.doi.org/10.1111/jpy.12025. PMid:27008383.
http://dx.doi.org/10.1111/jpy.12025...
). As with most other algae, freshwater Cladophora sp. removes nitrate and phosphate from water to make biomass and is primarily limited by phosphorous availability (Canale et al., 1982CANALE, R.P., AUER, M.T. and GRAHAM, J.M., 1982. Ecological studies and mathematical modeling of Cladophora in Lake Huron: 6. Seasonal and spatial Variation in Growth Kinetics. Journal of Great Lakes Research, vol. 8, no. 1, pp. 126-133. http://dx.doi.org/10.1016/S0380-1330(82)71950-1.
http://dx.doi.org/10.1016/S0380-1330(82)...
; Planas et al., 1996PLANAS, D., MABERLY, S.C. and PARKER, J.E., 1996. Phosphorus and nitrogen relationships of Cladophora glomerata in two lake basins of different trophic status. Freshwater Biology, vol. 35, no. 3, pp. 609-622. http://dx.doi.org/10.1111/j.1365-2427.1996.tb01772.x.
http://dx.doi.org/10.1111/j.1365-2427.19...
; Higgins et al., 2006HIGGINS, S.N., HECKY, R.E. and GUILDFORD, S.J., 2006. Environmental controls of Cladophora growth dynamics in Eastern Lake Erie: application of the Cladophora growth model (CGM). Journal of Great Lakes Research, vol. 32, no. 3, pp. 629-644. http://dx.doi.org/10.3394/0380-1330(2006)32[629:ECOCGD]2.0.CO;2.
http://dx.doi.org/10.3394/0380-1330(2006...
; 2008HIGGINS, S.N., MALKIN, S.Y., TODD HOWELL, E., GUILDFORD, S.J., CAMPBELL, L., HIRIART-BAER, V. and HECKY, R.E., 2008. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. Journal of Phycology, vol. 44, no. 4, pp. 1-16. http://dx.doi.org/10.1111/j.1529-8817.2008.00538.x. PMid:27041601.
http://dx.doi.org/10.1111/j.1529-8817.20...
; Malkin et al., 2009MALKIN, S.Y., SORICHETTI, R.J., WIKLUND, J.A. and HECKY, R.E., 2009. Seasonal abundance, community composition, and silica content of diatoms epiphytic on Cladophora glomerata. Journal of Great Lakes Research. vol. 35, pp. 199-205., Young et al.,2010YOUNG, E.B., TUCKER, R.C. and PANSCH, L.A., 2010. Alkaline phosphatase in freshwater Cladophora-epiphyte assemblages: regulation in response to phosphorus supply and localization. Journal of Phycology, vol. 46, no. 1, pp. 93-101. http://dx.doi.org/10.1111/j.1529-8817.2009.00782.x.
http://dx.doi.org/10.1111/j.1529-8817.20...
; Liu and Vyverman, 2015LIU, J., and VYVERMAN, W. 2015. Differences in nutrient uptake capacity of the benthic filamentous algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under varying N/P conditions. Bioresource Technology, vol. 179, pp. 234-242. http://dx.doi.org/10.1016/j.biortech.2014.12.028.
http://dx.doi.org/10.1016/j.biortech.201...
). Furthermore, Cladophora sp. has been effective in heavy metal removal from industrial liquid wastewater (Lee and Chang, 2011LEE, Y.-C. and CHANG, S.-P., 2011. The biosorption of heavy metals from aqueous solution by Spirogyra and Cladophora filamentous macroalgae. Bioresource Technology, vol. 102, no. 9, pp. 5297-5304. http://dx.doi.org/10.1016/j.biortech.2010.12.103. PMid:21292478.
http://dx.doi.org/10.1016/j.biortech.201...
; Ji et al., 2011JI, L., XIE, S., FENG, J., LI, Y. and CHEN, L., 2011. Heavy metal uptake capacities by the common freshwater green alga Cladophora fracta.Journal of Applied Phycology, vol. 24, no. 4, pp. 979-983. http://dx.doi.org/10.1007/s10811-011-9721-0.
http://dx.doi.org/10.1007/s10811-011-972...
; Abdel-Raouf et al., 2012ABDEL-RAOUF, N., AL-HOMAIDAN, A.A. and IBRAHEEM, I.B.M., 2012. Microalgae and wastewater treatment. Saudi Journal of Biological Sciences, vol. 19, no. 3, pp. 257-275. http://dx.doi.org/10.1016/j.sjbs.2012.04.005. PMid:24936135.
http://dx.doi.org/10.1016/j.sjbs.2012.04...
). Although, a Chilean environmental law regulates the discharge of organic wastewater into the environment, there are still an important number of small dairy industries that do not comply with the law, discharging wastewater directly into the environment (Baranao and Tapia, 2004BARAÑAO, P.A. and TAPIA, L.A., 2004. Tratamiento de las Aguas Servidas: Situación en Chile. Ciencia & Trabajo, no. 13, pp. 111-117.). Law enforcement is still soft, especially for small farmers, mainly because water treatments require the use of costly chemicals or advanced technology, which are difficult to afford (Dirección General de Aguas, (Chile, 2004CHILE. MINISTERIO DE OBRAS PÚBLICAS. DIRECCIÓN GENERAL DE AGUAS, 2004. Diagnótico y clasificación de los cursos y cuerpos de agua según objetivos de calidad. Chile: Cuenca del Río Itata.)). If the law is not enforced, the environment is subject to being damaged every day, and if it is enforced, small farmers might be fined. Therefore, the filamentous algae Cladophora sp. is a suitable organism to be used as a low-cost technology for the removal of organic nutrients from agricultural liquid waste from small dairy farms, primarily because positive net photosynthesis can be achieved at very low irradiance (Graham et al., 1982GRAHAM, J.M., AUER, M.T., CANALE, R.P. and HOFFMANN, J.P., 1982. Ecological Studies and Mathematical Modeling of Cladophora in Lake Huron: 4. Photosynthesis and respiration as functions of light and temperature. Journal of Great Lakes Research, vol. 8, no. 1, pp. 100-111. http://dx.doi.org/10.1016/S0380-1330(82)71948-3.
http://dx.doi.org/10.1016/S0380-1330(82)...
). Although the biological removal of nutrients is efficient and considered a low-cost technology/method, the use of filamentous algae has its limitations. One of these limitations is the amount of clean water needed for the dilution of the dark organic wastewater in order to allow the filamentous algae to grow. Nevertheless, filamentous algae like Cladophora sp. can be harvested and used as a secondary product for compost, biofertilizer, bioestimulants or as a good source of cellulose fibers, helping farmers obtain alternative resources (Wilkie and Mulbry, 2002WILKIE, A.C. and MULBRY, W.W., 2002. Recovery of dairy manure nutrients by benthic freshwater algae. Bioresource Technology, vol. 84, no. 1, pp. 81-91. http://dx.doi.org/10.1016/S0960-8524(02)00003-2. PMid:12137274.
http://dx.doi.org/10.1016/S0960-8524(02)...
; Ross et al., 2018ROSS, M.E., DAVIS, K., MCCOLL, R., STANLEY, M.S., DAY, J.G. and SEMIÃO, A.J.C., 2018. Nitrogen uptake by the macro-algae Cladophora coelothrix and Cladophora parriaudii: Influence on growth, nitrogen preference and biochemical composition. Algal Research, vol. 30, pp. 1-10. http://dx.doi.org/10.1016/j.algal.2017.12.005.
http://dx.doi.org/10.1016/j.algal.2017.1...
; Sucaldito and Camacho 2017SUCALDITO, M.V. and CAMACHO, D.H., 2017. Characteristics of unique HBr-hydrolyzed cellulose nanocrystals from freshwater green algae (Cladophora rupestris) and its reinforcement in starch-based film. Carbohydrate Polymers, vol. 169, pp. 315-323. http://dx.doi.org/10.1016/j.carbpol.2017.04.031. PMid:28504150.
http://dx.doi.org/10.1016/j.carbpol.2017...
).
2. Materials and Methods
2.1. Sample collection
Filamentous algae samples were collected within the urban perimeter of Chillán from a small pond located in the Ñuble Region, Chile. The filamentous algae are part of the metaphyton, therefore, it was collected manually with prehensile tools and by dragging it from the shore (Collins and Weber, 1978COLLINS, G.B. and WEBER, C.I., 1978. Phycoperiphyton (Algae) as Indicators of Water Quality. Transactions of the American Microscopical Society, vol. 97, no. 1, pp. 36. http://dx.doi.org/10.2307/3225682.
http://dx.doi.org/10.2307/3225682...
). The samples were transported to the laboratory in hermetically plastic bags where they were cleaned of leaf detritus and observed under an Olimpus CX31 optical microscope for identification. The algae were identified to the genus level using taxonomic keys for Chlorophycean algae (Graham et al., 2018GRAHAM, L.E., GRAHAM, J.M., WILCOX, L.W. and COOK, M.E., 2018. Algae. 3rd ed. Madison, WI: LJLM Press. 616 p.). Additionally, pictures of the algae were sent to phycology expert Dr. Linda Graham (UW-Madison, USA) for further identification confirmation.
2.2. Filtered pool water (FW)
Water from the pond where algae thrived naturally was sampled and filtered through a Whatman® filter N°1 to take out coarse leaf and insect detritus. Inorganic nutrients or bacterial consortium were not eliminated because it was desirable to preserve the natural habitat of the algae. Filtered water was used to make enough algal biomass and was also used as control.
2.3. Industrial Liquid Wastewater (ILW)
Wastewater was obtained at the end of summer 20 km north of Chillán, from an experimental dairy farm located on property owned by the University of Concepción for veterinary research. The milking procedure is rudimentary: untreated wastewater runs off into the fields and a nearby stream. The small farm has 40 milking cows and the entire milk yield is sold to a dairy company. Wastewater was collected in plastic bottles, carried immediately to the laboratory, and kept refrigerated at 4°C in the dark until it was ready to use the following day.
2.4. Sample treatment
Algae filaments were cleaned from leaf detritus and cultured at 18 °C in filtrated pond water (FW) to achieve greater algal biomass and preserve fresh samples by simulating its natural habitat. In addition, the algal filaments were cultivated in liquid agricultural wastewater (ILW) to verify their reproductive success and prove their effectiveness in the reduction of PO43- and NO3-.
2.5. Algae incubation
The initial inoculum was prepared by separating the algal mats using a sterile scalpel to obtain an even mix of algal filaments that were diluted with 100 mL of FW. ILW was diluted with FW at five different incremental ratios (Table 1). Each ILW:FW dilution was dispensed in 1000-mL flasks and inoculated with 1 mL of the solution of algal filaments, starting with an initial concentration of 1.0 mg*L-1 of Chlorophyll-a in all treatments in triplicates. The algal filaments grew for at least three days in every treatment for acclimatization. Chlorophyll-a was used to know the optimal ILW: FW dilution at which the algae could still thrive under minimum light intensity. The incubation period would be finished when chlorophyll-a would reach a similar or higher concentration value to that obtained in FW as the control.
Levels of chlorophyll-a (mg*L-1) (n=3) after 9 days of cultivation and light intensity that reached the algae at the culture solution. FW (filtered water), ILW (agricultural liquid waste).
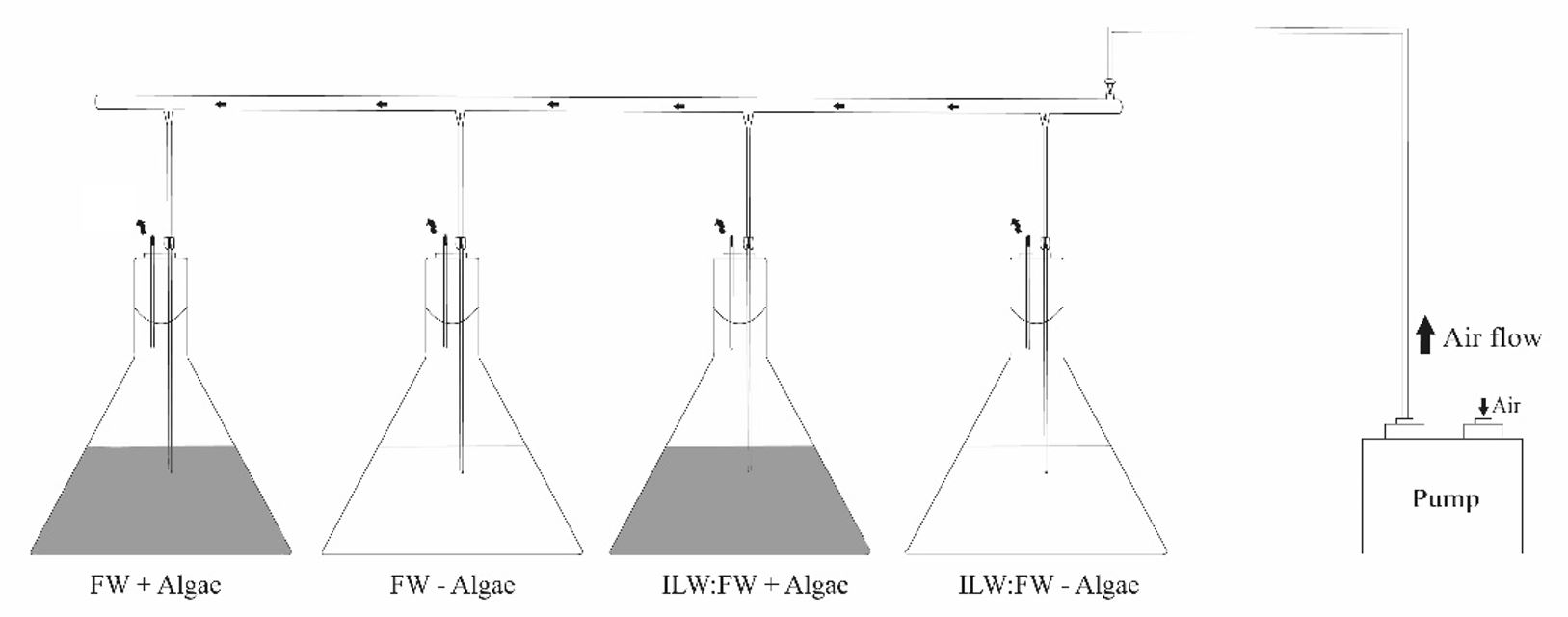
The flasks were illuminated with fluorescent white light at an intensity of 1667 µmol photons*m-2*s-1 Photosinthetically Active Radiation (PAR), with a photoperiod of 16:8 (L:D) and at a temperature of 18 °C during the whole incubation period. PAR light intensity was measured at every ILW:FW dilution using a Quantometer/Photometer LiCOR. The pH of the culture medium was 6.9, which matched the pH of the collected wastewater. The pH level was continuously monitored and did not change during the incubation period. A schematic figure of the system is shown (Figure 1).
A schematic figure of the treatments at the laboratory-scale experiments for nutrient removal using filamentous algae Cladophora.
Chlorophyll-a was measured every day in triplicate during the whole incubation period in all treatments. Measurements were done by spectrophotometry using the following equation: mg*L-1 = 11.6 (D.O. 665)-1.31 (D.O. 645)-0.14 (D.O. 630) (Hansmann, 1973HANSMANN, E., 1973. Pigment analysis In: J.R. STEIN, ed. Handbook of Phycological Methods. Cambridge: Cambridge University Press, pp. 359-368.).
2.6. Nutrient analyses
Nutrients such as PO43- and NO3- were measured in triplicate on days one and nine, following the methodology suggested by APHA (Eaton and Franson, 2005EATON, A. D. and FRANSON, M. A. H. (2005). Standard methods for the examination of water and wastewater 21st ed. Washington, D. C.: APHA-AWWA-WEF.). Statistical analyses were done using Duncan Test at the significant level of p≤ 0,05. All analyses were performed with Statistica 10.0.
3. Results
Filamentous algae collected from a freshwater pond were identified as belonging to the Division: Chlorophyta, Family: Cladophoraceae, Order: Cladophorales and Genus: Cladophora (Kützing, 1843KÜTZING, F. T. 1843. Phycologia generalis oder Anatomie, Physiologie und Systemkunde der Tange. Mit 80 farbig gedruckten Tafeln, gezeichnet und gravirt vom Verfasser. pp. [part 1]: [i]-xxxii, [1]-142, [part 2:] 143-458, 1, err.]. Leipzig: F.A. Brockhaus. pp. 1-80.). The filaments are septated and branched, chloroplasts are reticular with numerous pyrenoids. Morphological features and habitat are evidence that the algae belong to the genus Cladophora.
3.1. Chlorophyll-a
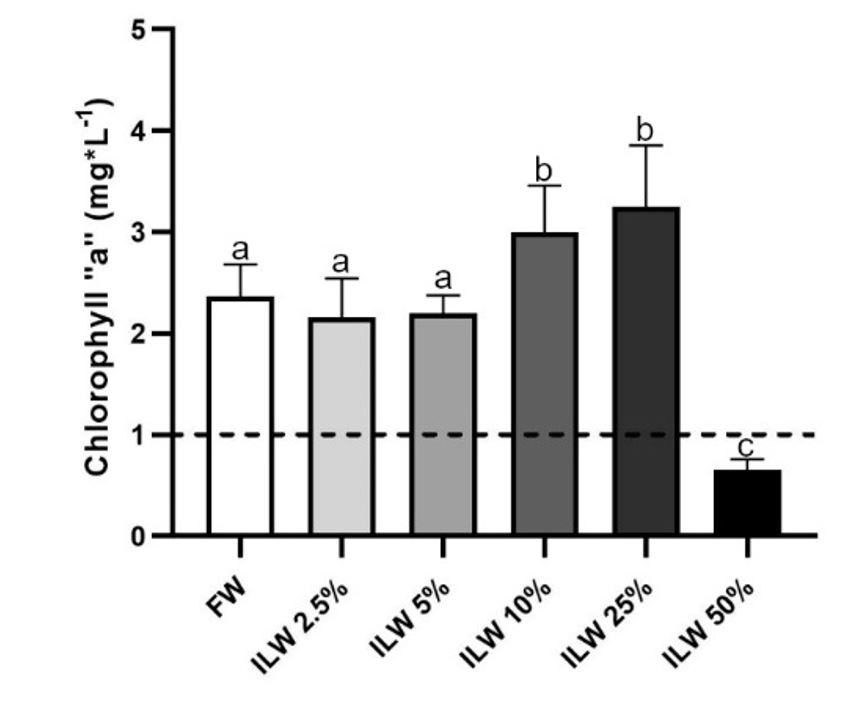
Chlorophyll-a measurements after 9 days of cultivation had a peak of 3.25 mg*L-1 at the ILW:FW (25.0:75.0) ratio when light intensity illuminating algae was 200 μmol photons*m-2*s-1. Meanwhile at the positive control (FW) the chlorophyll-a reached 2.63 mg*L-1 (Figure 2; Table 1) at day 9.
Concentration of chlorophyll “a” measured in filtered water (FW), and in a ILW:FW 2.5%, 5.0%, 10.0%, 25.0% and 50.0% ratios after nine days of cultivation. The punctuated line shows the initial concentration of chlorophyll-a in all treatments. Different letters indicate statistical differences among averages; p<0.05.
The algae grew in dilutions of ILW:FW 2.5%, 5.0% reaching levels of chlorophyll-a of 2.16, 2.19 mg*L-1 respectively, which is below the level of chlorophyll-a (2.63 mg*L-1) measured at the positive control. The ILW:FW 10.0% dilution rate had a final chlorophyll-a level of 2.99 mg*L-1 when light intensity measured inside each algal culture flask diminished to 300 μmol photons*m-2*s-1. The ILW:FW of 25% reached chlorophyll-a levels of 3.25 mg*L-1 when light intensity diminished to 200 μmol photons*m-2 *s-1. Finally, at the 50% dilution, chlorophyll-a dropped to 0.65 mg*L-1 when light intensity dropped to 80 μmol photons*m-2 *s-1 (Table 1).
3.2. Phosphate
The phosphate content of filtered water during the first incubation day was 21.2 µg*L-1. When algae were added, phosphate concentration dropped to 3 µg*L-1 on the ninth day, while treatment without algae dropped to 16 µg*L-1. In ILW:FW (25:75) ratio plus algae, the initial phosphate content on day one was 78.5 µg*L-1. When algae were added, phosphate concentration dropped to 9.7 µg*L-1 after nine days, while in the ILF:FW (25:75) treatment without algae phosphate concentration dropped to 70 µg*L-1.(Figure 3a, Table 2).
(a) initial and final (after 9 days of cultivation) concentration of phosphate in filtered water (FW) with (+) and without (-) Cladophora sp. and agricultural liquid waste (ILW:FW) at 25:75 ratio with (+) and without (-) Cladophora sp (b) initial and final (after 9 days of cultivation) concentration of nitrate in filtered water (FW) with and without Cladophora sp. and agricultural liquid waste (ILW:FW) at 25:75 ratio with (+) and without (-) Cladophora sp.
Concentration levels of phosphate (µg*L-1): Initial and final (after 9 days of cultivation) concentration of phosphate in filtered water (FW) with (+) and without (-) Cladophora sp. and agricultural liquid waste (ILW:FW) at 25:75 ratio with (+) and without (-) Cladophora sp. n=3. All values are significant p<0.05.
3.3. Nitrate
The initial nitrate content of filtered water was 13.7 mg*L-1. After nine days of incubation with algae, nitrate concentration dropped to 3.4 mg*L-1. However, with the FW treatment without algae, the nitrate concentration dropped to 10.1 mg*L-1 after nine days of incubation. In the treatment ILW:FW (25:75) ratio plus the algae inoculum, the initial nitrate content at day one was 164.8 mg*L-1, and after nine days dropped to 20.7 mg*L-1. Filtered water and ILW:FW (25:75) dilution rate minus algae treatments dropped to 150.0 mg*L-1 (Figure 3b, Table 3).
Concentration levels of nitrate (mg*L-1): Initial and final (after 9 days of cultivation) concentration of nitrate in filtered water (FW) with and without Cladophora sp. and agricultural liquid waste (ILW:FW) at 25:75 ratio with (+) and without (-) Cladophora sp. n=3 . All values are significant p<0.05.
4. Discussion
The use of algae for wastewater treatment has been shown an effective and efficient method to reduce nutrient loads and production costs (Kube et al., 2018KUBE, M., JEFFERSON, B., FAN, L. and RODDICK, F., 2018. The impact of wastewater characteristics, algal species selection and immobilization on simultaneous nitrogen and phosphorous removal. Algal Research, vol. 31, pp. 478-488. http://dx.doi.org/10.1016/j.algal.2018.01.009.
http://dx.doi.org/10.1016/j.algal.2018.0...
). The filamentous algae Cladophora sp. have very low nutritional and environmental requirements, which may result in a cost and productivity benefit (Sturm and Lamer, 2011STURM, B.S.M. and LAMER, S.L., 2011. An energy evaluation of coupling nutrient removal from wastewater with algal biomass production. Applied Energy, vol. 88, no. 10, pp. 3499-3506. http://dx.doi.org/10.1016/j.apenergy.2010.12.056.
http://dx.doi.org/10.1016/j.apenergy.201...
; Cydzik-Kwiatkowska and Zielińska, 2016CYDZIK-KWIATKOWSKA, A. and ZIELIŃSKA, M., 2016. Bacterial communities in full-scale wastewater treatment systems. World Journal of Microbiology & Biotechnology, vol. 32, no. 4, pp. 66. http://dx.doi.org/10.1007/s11274-016-2012-9. PMid:26931606.
http://dx.doi.org/10.1007/s11274-016-201...
).
The results indicate the optimal dilution rate for the efficient removal of nutrients by Cladophora is ILW:FW (25:75) ratio, given environmental parameters such as the ambient temperature of 18 °C and PAR irradiance of 200 µmol photons*m-2*s-1. Chlorophyll-a level showed that algae could thrive at 200 µmol photons*m-2*s-1, which is high in comparison with the reported range between 6-44 µmol photons*m-2*s-1 (Graham et al., 1982GRAHAM, J.M., AUER, M.T., CANALE, R.P. and HOFFMANN, J.P., 1982. Ecological Studies and Mathematical Modeling of Cladophora in Lake Huron: 4. Photosynthesis and respiration as functions of light and temperature. Journal of Great Lakes Research, vol. 8, no. 1, pp. 100-111. http://dx.doi.org/10.1016/S0380-1330(82)71948-3.
http://dx.doi.org/10.1016/S0380-1330(82)...
). Even though the flasks were illuminated with a constant PAR of 1667µmol photons*m-2*s-1, the dilutions were dark enough to reduce the PAR intensity as low as 80 µmol photons*m-2*s-1 at the (ILW:FW) 50:50 ratio, when chlorophyll-a reached the lowest value (0.657 mg*L-1). At that point, removal of nutrients also dropped in circa 80% of the samples. Light intensity and chlorophyll-a are interlinked in a way that can be used as indicators of the point at which algae will remove enough nitrate and phosphate to be discharged into the environment without exceeding the levels set in Chilean laws (Chile 2001CHILE. 2001. Ley n° 19.300. Decreto 90/00, sobre Bases Generales del Medio Ambiente. Diario Oficial de la República de Chile, Santiago, Chile, 7 de marzo., Law N° 19.300. Decree 90/00). In addition to nutrient removal from ILW, the possibility of separating and extracting the algal biomass opens the door to a world of opportunities from its use, with the potential to create a self-sufficient energy plant with recovery of nutrients such as nitrogen or phosphorus, decreasing the ecological debt (Santos and Pires, 2018SANTOS, F.M. and PIRES, J.C.M., 2018. Nutrient recovery from wastewaters by microalgae and its potential application as bio-char. Bioresource Technology, vol. 267, pp. 725-731. http://dx.doi.org/10.1016/j.biortech.2018.07.119. PMid:30082133.
http://dx.doi.org/10.1016/j.biortech.201...
).
Given the results of this research, agricultural wastewater may be treated in suitable mass waters like lagoons and wetlands using Cladophora sp. to remove organic phosphorus and nitrate (Zhu et al., 2018ZHU, H., LU, X. and DAI, H., 2018. Surface-flow constructed wetlands dominated by Cladophora for reclaiming nutrients in diffuse domestic effluent. Chemosphere, vol. 195, pp. 524-530. http://dx.doi.org/10.1016/j.chemosphere.2017.12.103. PMid:29277032.
http://dx.doi.org/10.1016/j.chemosphere....
). Since Cladophora sp. can thrive under low light intensities, lagoons should be no more than one meter deep to avoid anoxic bottom. Thus, it is suggested that freshwater should be introduced into the ILW until reaching a minimum light intensity of 200 µphotons*m-2*s-1. According to Kube et al. (2018)KUBE, M., JEFFERSON, B., FAN, L. and RODDICK, F., 2018. The impact of wastewater characteristics, algal species selection and immobilization on simultaneous nitrogen and phosphorous removal. Algal Research, vol. 31, pp. 478-488. http://dx.doi.org/10.1016/j.algal.2018.01.009.
http://dx.doi.org/10.1016/j.algal.2018.0...
, algae may continue removing nitrogen and phosphorus as long as phosphorus is not totally depleted, which makes algae a good candidate for removing both nutrients efficiently.
Some members of the genus Cladophora sp. must be anchored to a surface in their initial growth stages (Graham et al., 1982GRAHAM, J.M., AUER, M.T., CANALE, R.P. and HOFFMANN, J.P., 1982. Ecological Studies and Mathematical Modeling of Cladophora in Lake Huron: 4. Photosynthesis and respiration as functions of light and temperature. Journal of Great Lakes Research, vol. 8, no. 1, pp. 100-111. http://dx.doi.org/10.1016/S0380-1330(82)71948-3.
http://dx.doi.org/10.1016/S0380-1330(82)...
), needing only small water motion to thrive. The fact that Cladophora sp. grows in filaments, makes the task of harvesting easier by physical mechanisms such as hand-pulling. The harvested algae could then become valuable as compost in the same farm. Cladophora sp. requires a hard surface for initial attachment, warm water, some motion, and nutrients from the effluent from the milk processing site. In natural habitats Cladophora sp. is a foul-smelling nuisance algae that harbors many human pathogens (Ishii et al., 2006ISHII, S., YAN, T., SHIVELY, D.A., BYAPPANAHALLI, M.N., WHITMAN, R.L. and SADOWSKY, M.J., 2006. Cladophora (Chlorophyta) spp. Harbor Human Bacterial Pathogens in Nearshore Water of Lake Michigan. Applied and Environmental Microbiology, vol. 72, no. 7, pp. 4545-4553. http://dx.doi.org/10.1128/AEM.00131-06. PMid:16820442.
http://dx.doi.org/10.1128/AEM.00131-06...
). Therefore, it is suggested that Cladophora sp. must be handled carefully when extracting it from nearby ponds, including cleaning it from detritus while bacteria remains in place. The bacteria consortium plus the algae must be taken into consideration for the removal of nutrients as a whole, since they were never removed when the experiments were set up.
In Chile, the law to discharge effluents in lakes and streams states that the total phosphorous maximum allowed is 10 mg*L-1 without dilution, and 15 mg*L-1 with dilution. For nitrogen, the maximum allowed is 50 mg*L-1 without dilution, and 75 mg*L-1 with dilution (Chile, 2001CHILE. 2001. Ley n° 19.300. Decreto 90/00, sobre Bases Generales del Medio Ambiente. Diario Oficial de la República de Chile, Santiago, Chile, 7 de marzo., Law N° 19.300. Decree 90/00). Filamentous algae can be collected and should be spread on large surfaces for subsequent drying to avoid decomposition.
Although this study is considered preliminary because it reports on a simple laboratory-scale experiment on the possibility of treating dairy processing effluents, it shows the feasibility of nutrient removal using the filamentous algae Cladophora sp.
5. Conclusion
The freshwater filamentous algae Cladophora sp. reduces phosphorus and nitrate concentration levels at laboratory-scale. Thus, it is possible to use the algae for the treatment of dairy processing effluents. Nonetheless, pilot and real-scale experiments are needed to validate this biological wastewater treatment method. Consequently, the use of algae for wastewater treatment in small milk farms could help the farmers to comply with Chilean law and at the same time help to reduce eutrophication in nearby lakes and streams. Lastly, a recommendation to reassure ideal conditions of the treatment is intense monitoring of pathogens that can be present in the algal mats.
Acknowledgements
The authors wish to thanks the University of Bio-Bio for its support through the Grant Number: DIUBB 020507 3/R. Also would like to thanks the anonymous reviewers for its highly valuable comments that helped improve the article. Special thanks to Ms. Daniela Rivera, Dr. Andrés Rodriguez and Dr. Judith Hoffman for improvement of the written English in the manuscript.
-
(With 3 figures)
References
- ABDEL-RAOUF, N., AL-HOMAIDAN, A.A. and IBRAHEEM, I.B.M., 2012. Microalgae and wastewater treatment. Saudi Journal of Biological Sciences, vol. 19, no. 3, pp. 257-275. http://dx.doi.org/10.1016/j.sjbs.2012.04.005 PMid:24936135.
» http://dx.doi.org/10.1016/j.sjbs.2012.04.005 - ASLAN, S. and KAPDAN, I.K., 2006. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecological Engineering, vol. 28, no. 1, pp. 64-70. http://dx.doi.org/10.1016/j.ecoleng.2006.04.003
» http://dx.doi.org/10.1016/j.ecoleng.2006.04.003 - BARAÑAO, P.A. and TAPIA, L.A., 2004. Tratamiento de las Aguas Servidas: Situación en Chile. Ciencia & Trabajo, no. 13, pp. 111-117.
- CANALE, R.P., AUER, M.T. and GRAHAM, J.M., 1982. Ecological studies and mathematical modeling of Cladophora in Lake Huron: 6. Seasonal and spatial Variation in Growth Kinetics. Journal of Great Lakes Research, vol. 8, no. 1, pp. 126-133. http://dx.doi.org/10.1016/S0380-1330(82)71950-1
» http://dx.doi.org/10.1016/S0380-1330(82)71950-1 - CHILE. MINISTERIO DE OBRAS PÚBLICAS. DIRECCIÓN GENERAL DE AGUAS, 2004. Diagnótico y clasificación de los cursos y cuerpos de agua según objetivos de calidad Chile: Cuenca del Río Itata.
- CHILE. 2001. Ley n° 19.300. Decreto 90/00, sobre Bases Generales del Medio Ambiente Diario Oficial de la República de Chile, Santiago, Chile, 7 de marzo.
- COLLINS, G.B. and WEBER, C.I., 1978. Phycoperiphyton (Algae) as Indicators of Water Quality. Transactions of the American Microscopical Society, vol. 97, no. 1, pp. 36. http://dx.doi.org/10.2307/3225682
» http://dx.doi.org/10.2307/3225682 - CYDZIK-KWIATKOWSKA, A. and ZIELIŃSKA, M., 2016. Bacterial communities in full-scale wastewater treatment systems. World Journal of Microbiology & Biotechnology, vol. 32, no. 4, pp. 66. http://dx.doi.org/10.1007/s11274-016-2012-9 PMid:26931606.
» http://dx.doi.org/10.1007/s11274-016-2012-9 - DODDS, W.K. and GUDDER, D.A., 1992. The ecology of Cladophora.Journal of Phycology, vol. 28, no. 4, pp. 415-427. http://dx.doi.org/10.1111/j.0022-3646.1992.00415.x
» http://dx.doi.org/10.1111/j.0022-3646.1992.00415.x - EATON, A. D. and FRANSON, M. A. H. (2005). Standard methods for the examination of water and wastewater 21st ed. Washington, D. C.: APHA-AWWA-WEF.
- GRAHAM, J.M., AUER, M.T., CANALE, R.P. and HOFFMANN, J.P., 1982. Ecological Studies and Mathematical Modeling of Cladophora in Lake Huron: 4. Photosynthesis and respiration as functions of light and temperature. Journal of Great Lakes Research, vol. 8, no. 1, pp. 100-111. http://dx.doi.org/10.1016/S0380-1330(82)71948-3
» http://dx.doi.org/10.1016/S0380-1330(82)71948-3 - GRAHAM, L.E., GRAHAM, J.M., WILCOX, L.W. and COOK, M.E., 2018. Algae 3rd ed. Madison, WI: LJLM Press. 616 p.
- HANSMANN, E., 1973. Pigment analysis In: J.R. STEIN, ed. Handbook of Phycological Methods Cambridge: Cambridge University Press, pp. 359-368.
- HIGGINS, S.N., HECKY, R.E. and GUILDFORD, S.J., 2006. Environmental controls of Cladophora growth dynamics in Eastern Lake Erie: application of the Cladophora growth model (CGM). Journal of Great Lakes Research, vol. 32, no. 3, pp. 629-644. http://dx.doi.org/10.3394/0380-1330(2006)32[629:ECOCGD]2.0.CO;2
» http://dx.doi.org/10.3394/0380-1330(2006)32[629:ECOCGD]2.0.CO;2 - HIGGINS, S.N., MALKIN, S.Y., TODD HOWELL, E., GUILDFORD, S.J., CAMPBELL, L., HIRIART-BAER, V. and HECKY, R.E., 2008. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. Journal of Phycology, vol. 44, no. 4, pp. 1-16. http://dx.doi.org/10.1111/j.1529-8817.2008.00538.x PMid:27041601.
» http://dx.doi.org/10.1111/j.1529-8817.2008.00538.x - HIGGINS, S.N., TODD HOWELL, E., HECKY, R.E., GUILDFORD, S.J. and SMITH, R.E., 2005. The wall of green: The status of Cladophora glomerata on the northern shores of Lake Erie’s eastern basin, 1995-2002. Journal of Great Lakes Research, vol. 31, no. 4, pp. 547-563. http://dx.doi.org/10.1016/S0380-1330(05)70283-5
» http://dx.doi.org/10.1016/S0380-1330(05)70283-5 - ISHII, S., YAN, T., SHIVELY, D.A., BYAPPANAHALLI, M.N., WHITMAN, R.L. and SADOWSKY, M.J., 2006. Cladophora (Chlorophyta) spp. Harbor Human Bacterial Pathogens in Nearshore Water of Lake Michigan. Applied and Environmental Microbiology, vol. 72, no. 7, pp. 4545-4553. http://dx.doi.org/10.1128/AEM.00131-06 PMid:16820442.
» http://dx.doi.org/10.1128/AEM.00131-06 - JI, L., XIE, S., FENG, J., LI, Y. and CHEN, L., 2011. Heavy metal uptake capacities by the common freshwater green alga Cladophora fracta.Journal of Applied Phycology, vol. 24, no. 4, pp. 979-983. http://dx.doi.org/10.1007/s10811-011-9721-0
» http://dx.doi.org/10.1007/s10811-011-9721-0 - KUBE, M., JEFFERSON, B., FAN, L. and RODDICK, F., 2018. The impact of wastewater characteristics, algal species selection and immobilization on simultaneous nitrogen and phosphorous removal. Algal Research, vol. 31, pp. 478-488. http://dx.doi.org/10.1016/j.algal.2018.01.009
» http://dx.doi.org/10.1016/j.algal.2018.01.009 - KÜTZING, F. T. 1843. Phycologia generalis oder Anatomie, Physiologie und Systemkunde der Tange. Mit 80 farbig gedruckten Tafeln, gezeichnet und gravirt vom Verfasser. pp. [part 1]: [i]-xxxii, [1]-142, [part 2:] 143-458, 1, err.] Leipzig: F.A. Brockhaus. pp. 1-80.
- LEE, Y.-C. and CHANG, S.-P., 2011. The biosorption of heavy metals from aqueous solution by Spirogyra and Cladophora filamentous macroalgae. Bioresource Technology, vol. 102, no. 9, pp. 5297-5304. http://dx.doi.org/10.1016/j.biortech.2010.12.103 PMid:21292478.
» http://dx.doi.org/10.1016/j.biortech.2010.12.103 - LESTER, W.W., ADAMS, M.S. and FARMER, A.M., 1988. Effects of light and temperature on photosynthesis of the nuisance alga Cladophora glomerata (L.) Kütz. The New Phytologist, vol. 109, no. 1, pp. 53-58. http://dx.doi.org/10.1111/j.1469-8137.1988.tb00218.x
» http://dx.doi.org/10.1111/j.1469-8137.1988.tb00218.x - MALKIN, S.Y., SORICHETTI, R.J., WIKLUND, J.A. and HECKY, R.E., 2009. Seasonal abundance, community composition, and silica content of diatoms epiphytic on Cladophora glomerata Journal of Great Lakes Research vol. 35, pp. 199-205.
- LIU, J., and VYVERMAN, W. 2015. Differences in nutrient uptake capacity of the benthic filamentous algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under varying N/P conditions. Bioresource Technology, vol. 179, pp. 234-242. http://dx.doi.org/10.1016/j.biortech.2014.12.028
» http://dx.doi.org/10.1016/j.biortech.2014.12.028 - PLANAS, D., MABERLY, S.C. and PARKER, J.E., 1996. Phosphorus and nitrogen relationships of Cladophora glomerata in two lake basins of different trophic status. Freshwater Biology, vol. 35, no. 3, pp. 609-622. http://dx.doi.org/10.1111/j.1365-2427.1996.tb01772.x
» http://dx.doi.org/10.1111/j.1365-2427.1996.tb01772.x - ROSS, M.E., DAVIS, K., MCCOLL, R., STANLEY, M.S., DAY, J.G. and SEMIÃO, A.J.C., 2018. Nitrogen uptake by the macro-algae Cladophora coelothrix and Cladophora parriaudii: Influence on growth, nitrogen preference and biochemical composition. Algal Research, vol. 30, pp. 1-10. http://dx.doi.org/10.1016/j.algal.2017.12.005
» http://dx.doi.org/10.1016/j.algal.2017.12.005 - SANTOS, F.M. and PIRES, J.C.M., 2018. Nutrient recovery from wastewaters by microalgae and its potential application as bio-char. Bioresource Technology, vol. 267, pp. 725-731. http://dx.doi.org/10.1016/j.biortech.2018.07.119 PMid:30082133.
» http://dx.doi.org/10.1016/j.biortech.2018.07.119 - SHIN, H.S. and LEE, S.M., 1998. Removal of Nutrients in Wastewater by using Magnesium Salts. Environmental Technology, vol. 19, no. 3, pp. 283-290. http://dx.doi.org/10.1080/09593331908616682
» http://dx.doi.org/10.1080/09593331908616682 - STURM, B.S.M. and LAMER, S.L., 2011. An energy evaluation of coupling nutrient removal from wastewater with algal biomass production. Applied Energy, vol. 88, no. 10, pp. 3499-3506. http://dx.doi.org/10.1016/j.apenergy.2010.12.056
» http://dx.doi.org/10.1016/j.apenergy.2010.12.056 - SUCALDITO, M.V. and CAMACHO, D.H., 2017. Characteristics of unique HBr-hydrolyzed cellulose nanocrystals from freshwater green algae (Cladophora rupestris) and its reinforcement in starch-based film. Carbohydrate Polymers, vol. 169, pp. 315-323. http://dx.doi.org/10.1016/j.carbpol.2017.04.031 PMid:28504150.
» http://dx.doi.org/10.1016/j.carbpol.2017.04.031 - WHITTON, B.A., 1970. Biology of Cladophora in freshwaters. Water Resources, vol. 4, pp. 457-476.
- WILKIE, A.C. and MULBRY, W.W., 2002. Recovery of dairy manure nutrients by benthic freshwater algae. Bioresource Technology, vol. 84, no. 1, pp. 81-91. http://dx.doi.org/10.1016/S0960-8524(02)00003-2 PMid:12137274.
» http://dx.doi.org/10.1016/S0960-8524(02)00003-2 - YOUNG, E.B., TUCKER, R.C. and PANSCH, L.A., 2010. Alkaline phosphatase in freshwater Cladophora-epiphyte assemblages: regulation in response to phosphorus supply and localization. Journal of Phycology, vol. 46, no. 1, pp. 93-101. http://dx.doi.org/10.1111/j.1529-8817.2009.00782.x
» http://dx.doi.org/10.1111/j.1529-8817.2009.00782.x - ZHU, H., LU, X. and DAI, H., 2018. Surface-flow constructed wetlands dominated by Cladophora for reclaiming nutrients in diffuse domestic effluent. Chemosphere, vol. 195, pp. 524-530. http://dx.doi.org/10.1016/j.chemosphere.2017.12.103 PMid:29277032.
» http://dx.doi.org/10.1016/j.chemosphere.2017.12.103 - ZULKIFLY, S.B., GRAHAM, J., YOUNG, E.B., MAYER, R.J., PIOTROWSKI, M.J., SMITH, I. and GRAHAM, L.E., 2013. The genus Cladophora Kützing (Ulvophyceae) as a globally distributed ecological engineer. Journal of Phycology, vol. 49, no. 1, pp. 1-17. http://dx.doi.org/10.1111/jpy.12025 PMid:27008383.
» http://dx.doi.org/10.1111/jpy.12025
Publication Dates
-
Publication in this collection
10 Aug 2020 -
Date of issue
Jul-Sep 2021
History
-
Received
30 May 2019 -
Accepted
20 Feb 2020