Self-sufficiency and innovation in vaccine production and public health
Isaias Raw; Hisako G. Higashi
ABSTRACT
Butantan creates new technologies and industrial processes for vaccines and other immunobiologicals, supplying 150 million doses of antigen vaccines per year, which constitute 82% of all vaccines in the country, at affordable prices which are provided free to all children and older adults. New developments include a pertussis vaccine that can be produced at the same price as the traditional vaccine by a process that also yields an adjuvant that increases the efficacy of seasonal and avian influenza vaccines four-fold, reducing cost and increasing plant capacity; a technology which results in the highest industrial yield of human rabies vaccine to date, a combined vaccine for use in maternity wards for BCG-hepatitis B and pertussis and a lung surfactant that will decrease prenatal mortality not solved with vaccines. In collaboration with NIH, Path and PDVI, Butantan is beginning production and assay of rotavirus and dengue vaccines.
Keywords: Vaccines, Pertussis, Adjuvant, Rabies, Dengue, Rota, Surfactant.
Why produce vaccines?
SELF-SUFFICIENCY as a security factor for public health became evident in 1984, when the National Quality Control Institute demonstrated that snakebite serums and others produced in Brazil were, because of low titres and high contamination by bacteria and fungi, unsuitable for human use, creating an climate of insecurity, given that snakebite serums must be produced specifically for the local species. The only private producer, a multinational company that had purchased the Instituto Pinheiros, faced with the fact that it would have to renew its technology and reformulate its installations to meet Good Manufacturing Practices standards, preferred to leave the country. The government-owned producers were mobilized to produce more serums of better quality, with financial assistance from the Ministry of Health. In an emergency measure, Butantan installed a plant with new technology, and most of the government producers desisted. Similar developments took place in Africa and part of Asia, where the large companies that supply snakebite serums, given the need to invest without having profitable returns, simply abandoned production, leaving the population unprotected.
This was repeated with the sole producer of human albumin, which produced in deplorable conditions and without any reaction. Biobraz closed, leaving Brazil dependent on imported insulin. We also witnessed the abandonment of antibiotic plants, which made the country even more vulnerable.
A new threat to public health arose with the avian flu, when producers of flu vaccines declared at a World Health Organization Meeting that they would be compelled by local governments to reserve the vaccine against a pandemic, which has 80 micrograms of antigen, for their own populations, somehow imagining that they could survive without the rest of humanity. Butantan took the initiative, and in very short time installed a pilot plant to begin production of this vaccine, obtaining the vaccinal virus for the H5N1
Influenza from Vietnam (WHO) and from Indonesia (CDC Centers for Disease Control and Prevention), whose virus is now monopolized by the country of origin, which also must imagine it can survive alone or win part of the big business that the anticipated sale of the vaccine, at US$100 per dose, generates.
Public health cannot remain at the whim of profits, not even if limited to the population that can pay for the vaccines. As Japan demonstrated, the seasonal flu is disseminated by children who bring the virus home from school. Protection for families depends on the protection of those with whom their children enter in contact! Once these children are vaccinated, they cannot pass the virus to their families, reducing the frequency of pneumonia, without the government having to spend millions of dollars each year on a poorly effective multivalent vaccine. Nor will the children develop middle ear infections, which can lead to childhood deafness.
The Pasteur model
Louis Pasteur (1822-1895), invented the public laboratory, it was a foundation that developed, produced and reapplied its income to continue research and produce. One hundred Pasteur Institutes were created in developing countries. By the 1980s most of them were not operating. The legal structure that permitted economic self-sufficiency was corroding. For their survival, these Institutes needed to modernize and expand production, they needed to have a basic research sector, which existed in Brazil, where Fiocruz and Butantan are research institutes. They needed to invest in development. Since 1985, Butantan implanted the Biotechnology Center, where twenty doctors and 50 students were dedicated to research development, which the Bioindustrial Division transformed into products for society.
We need a reserve of supplies for the Ministry of Health that allows it to pay the prices it determines and pay when it can. The Ministry does not have resources to finance production that must be initiated eight to ten months before supply, which therefore falls in the following fiscal year, and is subject to tempestuous budget approvals (officially the Ministry ordered the seasonal flu vaccine in 2008), only after it received and used the vaccine.
Most of the government-owned producers entered in crisis, terminated production and became bottlers of vaccines imported in bulk. This "Coca-Cola model" - buy syrup, dilute and bottle - does not allow the Institutes to learn to produce, much less gain technological independence, which is the foundation for innovation. They are hostage to the large companies that supply in bulk and that in fact set the supply prices for the Ministry. Rarely do they acquire technology, and when they do, it is usually as a "black-box", which does not allow innovation. They are dependent on strategic inputs that allow the technology supplier to be the true owner of this technology and control how much is produced, the price to be paid by the Ministry, and therefore, the royalties that are due to them.
In contrast, Butantan developed its own technology and commands production. This control required designing and building new production plants. Butantan constantly teaches Brazilian builders what is needed, such as filtered air to maintain sterility and even teaches Brazilian companies to produce equipment that had always been imported.
Seasonal and avian flu
In 2000, Butantan cooperated with the Ministry of Health to introduce the flu vaccine for people older than 60. It reached an agreement under exceptional conditions that would never be repeated for technology transfer, strictly limited to the purchase of the vaccine in bulk until its factory was completed. This agreement had two important consequences. The first was that we negotiated a special price that resulted in savings of nearly US$300 million, which we transferred to the Ministry, lowering the price of the vaccine, without leaving any debt. Second, by using European technology, we resolved the problem caused by the fact that it was impossible to test the vaccine for that year this was conducted by the WHO which supplies the strains a few months before vaccination. The Ministry of Health invested approximately R$34 million in the flu vaccine plant and the Secretary of Health some R$40 million. Unfortunately, the inexperience of the builder and its associates caused a delay of nearly three years for the plant to be ready for validation. This agreement allowed vaccinating, an average of more than 80% of the population older than 60 each year, beginning with 7.5 million doses and in 2007 reaching nearly 18.3 million, in a partnership between the Ministry, Butantan and the states and municipalities. The incidence of flu decreased significantly as well as hospitalizations for pneumonia.
When we decided to produce the avian flu vaccine, in three months we built a plant to meet the strategic demand of 200,000 doses per year, which would be sufficient to vaccinate a group that entered the country and was exposed to this pandemic form. The Ministry of Health and the financing agencies could not react with the same speed and the Foundation initiated the necessary investment.
In 2007, the U.S. government announced that it would finance Butantan, but the funds wound up being delivered to the WHO, which chose Butantan and four other potential vaccine producers from developing countries. The project began production in 2008.
DTP, a basic vaccine
The combined diphtheria-tetanus-whopping cough vaccine (DTP) was developed in the 1930's and became the foundation for creating tetravalent, pentavalent and hexavalent vaccines. The use of multiple vaccines simplifies administration and reduces the significant cost of the syringes. To allow production under Good Manufacturing Practices (GMP), we had to build new installations, take advantage of "skeletons" that were abandoned for decades, new laboratories were built, and use new technology developed at Butantan, which demanded a new generation of equipment. A center was built for formulation and bottling, which washed and sterilized flasks, transferred the vaccines and then sealed and labeled, allowing the production of 28 thousand flasks per hour. With five to ten doses per flask, we could reach more than two million doses per day!
Since 1990, nearly 400 million doses of this vaccine have been produced and administered to practically all children younger than two. By choosing a strain of Bordetella pertussis and using a new inactivation process, we were able to practically eliminate the serious collateral effects. The three childhood infections practically disappeared.
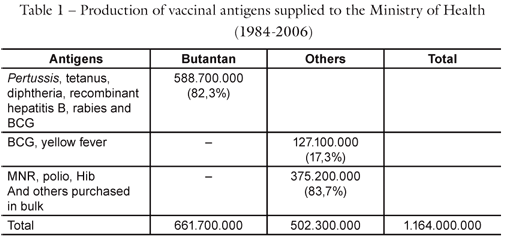
Three plants were built to produce these vaccines, which were totally self-contained, with capacity to meet Brazilian demand of nearly 25 million doses per year, for four doses per child and to supply the vaccines needed each year at 25 thousand vaccination posts in the country. In addition to the children's vaccine, we produced the Dual dT vaccine for Adult Use (diphtheria-tetanus), which in 2004 allowed revaccinating all of the 20 million people older than 60, given that diphtheria began to attack the elderly. Butantan alone produced 20 million, while in the following years this dropped to only two million. This was possible thanks to improvements in production, which is only possible with the implantation of technology. Butantan can now produce up to 180 million DTP Triple vaccines (diphtheria, tetanus, pertussis) (or its combinations) per year, and began exports.
Hepatite B and haemophilus b
In 1995, we began to produce the recombinant vaccine against hepatitis B with technology developed at the Institute by a contracted researcher. The vaccine was immediately tested and approved at the University of Londrina, but had to wait five years and undergo five thousand trials to be recognized as efficient as the comparable vaccine. Nearly 160 million doses already produced attest to the effectiveness and safety. There are now dozens of producers of the hepatitis B vaccine, and the cost dropped from US$ 20 to US$ 0.22! At private clinics in Brazil or the United States the vaccine continues to cost nearly US$50.00 per dose!
An obvious development would be the combination DTP-hepatitis B, adopted by most countries. Biomanguinhos decided to purchase the vaccine against haemophilus b in bulk and we suspended the development of our Hib vaccine (Haemophilus influenzae b). We ceded DTP to release the vaccine DTP-Hib, leaving the hepatitis B vaccine separate.
We returned to developing the Hib vaccine and began assembling a plant for its production. The Hib vaccine, which conjugates the polysaccharide of this bacteria with the tetanic anatoxin, is definitively an effective vaccine. Butantan is one of the largest producers of tetanic anatoxin, which is used for the conjugate vaccine. With this improvement, we have installed capacity to produce 180 million doses per year. Butantan will soon begin producing the DTP-hepatitis B vaccine that can be combined at public health clinics with the Hib, from Butantan or from Biomamguimhos, making the vaccine pentavalent. Butantan does not have export restrictions on its vaccine.
Because of the lack of competition, for more than a decade the price has remained above US$ 2,50 and the Hib vaccine is now used by only one of five children. In Africa, meningitis A is predominant, which is not common in Brazil. The vaccine conjugates the polysaccharide of meningitis A with the tetanic anatoxin with a very low yield. The Program for Appropriate Technology in Health (PATH) decided to invest in improving production and reached a process that allowed serving Africa (specifically by an Indian company) at a cost of only US$ 0.50. The basic technology to produce the Hib and meningitis A vaccines are similar, and the vaccination will reach more children if, given market rules, production increases, if it is offered as it should be at a price below US$ 1. This is what Butantan intends to do, meeting the requests of Asian and African countries.
The Gates Foundation imagined that US$ 5 would be sufficient to offer basic vaccines for the children of much poorer countries, and that by increasing the market of large producers, the price of the vaccine for developing countries would be reduced. It began to purchase the vaccines and offer them to developing countries and, with the results obtained, the governments wound up assuming, with their own resources, the public vaccination. It is increasingly evident that, despite the increased market, the prices did not fall, and that the companies would not invest based on the expectation that the market would be maintained after the Gates-World Bank- Rockefeller Foundation stopped financing. For the first time, ideas were suggested at international meetings to re-establish government production of vaccines, which countered the historic inability of state-owned companies to produce with quality and safety. They were notorious for their lack of managerial capacity and in particular, for an inability to innovate and develop new vaccines. The large companies changed their focus to high cost vaccines, which would never be available for the population and governments with limited resources. Even the Pan American Health Organization (PAHO) and the World Health Organization (WHO) began to promote the purchase of these vaccinations at unviable costs, and now promote the vaccine for rotavirus at US$ 15 or more per child and the 7-valent pneumococcal conjugate vaccine at US$ 159 for three doses, and even the HPV vaccine at US$600 or more for each girl or woman! Private companies, by nature, give priority to profits, not public health. Evidence of this is that one of the countries with the largest number of competent producers of vaccines, India, offers its products for export and has been able to produce quality vaccines for the rotating funds of Unicef and PAHO, and because of the competition, reduce costs. Some now produce for the large multinational companies. Nevertheless, a simple analysis shows that India does not have priority access to these vaccines, which is evident by the fact that it is the world's last region affected by polio.
New pertussis and flu vaccine
The DTP vaccine from Butantan has proved to be effective and safe, which was not the case in Japan, where the pertussis vaccine was highly toxic. H. Sato, in Japan, isolated two essential proteins for immunizationa against pertussis. The large companies increased from to three to five the number of vaccinal antigens without a strong benefit. The new vaccines, with isolated proteins, denominated acellular (aDTP), have a lower frequency of serious collateral effects and the same effectiveness as the classic vaccine (wDTP). The cost of wDTP, at the PAHO rotating fund is US$ 0.15 per dose. The aDTP vaccine costs US$ 8.15. Without any cost-benefit perspective, some societies, accepting the propaganda of the producers, recommended substituting wDTP for aDTP, which for Brazil would represent a cost of US$ 130.44 million! In November 2007, the PAHO and WHO met to declare that this substitution has no justifiable benefit.
Butantan, always concerned with cost-benefit factors and economizing public funds at the Ministry for the introduction of new vaccines, developed a technology that allowed removing from Bordetella lipopolysaccharide (LPS), a substance that links to Toll-Like Receptor 4 from the cell membrane, triggering an inflammatory response. The new pertussis, whose clinical trial was conducted by the Pediatrics Department of the University of Campinas (Unicamp), and which we call Plow, does not increase the cost of the vaccine, which remains like DTP at US$ 0.15 per dose. An agreement allowed the Netherland Vaccine Institute, pioneer in industrial production of vaccines, to use Butantan's technology.
With a current capacity to produce 180 million doses of the DPTlow vaccine, we accumulated 72 million milligrams of LPS. We developed a method to hydrolyze the LPS, producing 36 million milligrams of monophosphoryl Lipid A (MPLA), which unlike, LPS, when linking to the Toll-Like receptor 4, does not trigger, from the production of cytocins, the inflammatory reaction, but stimulates, by means of production of interferons, the activation of the T linphocits.
The pertussis vaccine, in the wDTP, is tested before it is liberated for use, by the induction of protection against a strain of Bordetella, chosen for its virulence. It is injected directly into mice cerebrum, killing those which are not protected. We discovered that one of the subunits of the pertussis toxin, whose gene was introduced in the BCG vaccine, was capable of protecting the mice against the virulent Bordetella strain, without producing antibodies. Contrary to what was believed, it is not the antibodies induced by the vaccine, but the cellular immunity it induces that creates the immunological memory.
Butantan has been studying the role of cellular immunity induced by the flu vaccine. A new adjuvant was developed and tested in mice, which includes MPLA and aluminum hydroxide, seeking to increase in the flu vaccine the humoral and cellular response that allows a four-fold reduction in the quantity of antigens in the vaccine formulation, permitting increased production at the seasonal flu vaccine plant from 20 million in four months to 80 million, obviously reducing the cost of the vaccine.
The choice made in 2008 of the strains of the seasonal flu vaccine for the Southern hemisphere created problems, because of the low productivity and low immunogenicity. For the H5N1 vaccine for the pandemic, it is already known that the vaccines built by reverse genetic engineering, producing a DNA using the viral RNA as a mold, and making some modifications to attenuate the virus, result in a vaccine that demands 80 micrograms per dose (the seasonal vaccine uses only 15 micrograms for each one of the three strains). It is essential to use an adjuvant to reduce the quantity of the H5N1 antigen per dose of vaccine, which multiplies the installed capacity that would be mobilized in case of a pandemic. Upon testing the adjuvant MPA (produced from the LPS of a Salmonella) in mice, the need was found to add 100 micrograms. When emulsified with squalene, the quantity to be added was 100 times less. Squalene is an oily compound isolated from sharks, which are now threatened with extinction and which will have to be substituted by a synthetic compound, which is much more expensive. A child vaccinated with wDTP receives, with each dose, 400 micrograms of LPS, corresponding to nearly 200 micrograms of MPLA, which usually does not cause serious collateral effects.
An important discovery was recently made. The collateral effects of the pertussis vaccine, which occur in one of every two thousand vaccinated children, are due to a genetic defect that affects a a sodium transporter of the synapses. At this time it is impossible to detect this mutation in the children who must be vaccinated. This does not decrease the value of the DTPlow, which has proved to be an effective and less toxic vaccine without an increase in costs.
One of the problems is protecting babies against whopping cough before the fourth month, when they receive the second dose of DTP. We were able to create a vaccine to be administered in maternity wards, substituting the BCG for a BCG that has included in its genome the S1 subunit of the pertussis toxin which is associated to the hepatitis B vaccine, protecting the baby against the possible transmission of hepatitis B from contact with maternal blood. There are preliminary indications that the S1 subunit has adjuvant effect and should stimulate the protection given by the BCG against tuberculosis and, probably, as onco-BCG.
Vaccine against rabies and leishmaniasis
For decades, Butantan produced a vaccine against rabies by inoculating the virus in the cerebrum of one-day old mice (before they begin to produce myelin). The vaccine was a portion of macerated cerebrum. It did not guarantee reliability and was particularly dangerous, because small quantities of cerebral mice myelin produced antibodies for the myelin of the person vaccinated, leading to Guillain-Barré syndrome. As we did with the BCG plant, we simply stopped production of this vaccine and developed VERO cells, achieving the highest reported yield per liter of the culture. We already meet demand in Brazil and are building a larger plant to meet demand for countries that do not have production capacity.
A derivative of this technology is the anti canine-rabies vaccine, produced in BHK cells (used to immunize horses to produce the anti-rabies serum) which must substitute the Fuenzalida Palácios vaccine, which the Ministry of Health purchased. This vaccine and the vaccine for human use and the anti-rabies serum are essential for meeting demand in Asia and Africa.
In collaboration with the University of Washington (the Infectious Disease Research Institute, IDRI, in Seattle), we are developing a combined rabies, leshmaniasis vaccine for dogs, which would block the transmission of two diseases to humans. These studies have the participation of the Federal University of Minas Gerais and of Fiocruz in Bahia.
"Translational" Studies public-private partnerships
At times a word becomes associated to a trend and acquires a new connotation. "Translational" research involves the transfer of research from the laboratory to clinical trial and production. The use of this neologism became obvious to us when we assumed the renovation of production of immunobiologicals, which society and the government itself demand: products made available to society to resolve individual and public health problems. This process had to be reinvented in a country where research gives priority to publication, leaving it up to industry to "translate" the knowledge into application. If this pragmatic vision destroys basic research and the ability of the scientific community to map new knowledge and where it is taken, we will not escape from underdevelopment. We cannot platonically accompany research that advances at frightening speed, without our professors and researchers having the liberty to develop original scientific research. Brazil has achieved important progress that should accelerate rapidly. Nevertheless, we cannot be content simply with scientific publications in high-level journals. They do not lead to "translation". The teaching staff at universities do not develop technology, nor do they have the ability or the facilities to conceive an efficient, low cost and quality industrial production process capable of meeting societal demands. This role is being substituted by the patent and by the intention of the government to share income from patents with researchers. Contrary to what takes place in the First World, where part of the public funding generates at least "proof of principles" as a basis for their transformation in product, private companies in Brazil do not have the competence, appetite, financial or human resources to conduct this phase that is essential to development. In comparison with advanced countries, the funding for basic research and for the small staff of researchers are relatively greater (when considering salaries, infrastructure and frequently equipment and breeding facilities are paid by the institutional budget). In a simplistic vision, it is enough to offer resources and the private company will do the development, using the deposited patents. Private and even government companies are looking for black boxes and answers that researchers don't have such as technological processes from which they can obtain raw materials; suppliers of industrial equipment; how to build the black box; the demand for energy and water and where to find trained technicians.
This is the complex that we have to develop and implant in the BioIndustrial Division of Butantan. The two dozen researchers at the Biotechnology Center attract dozens of graduate students, without whom the research would not advance. They are ready to give priority to projects with social impact, even while they have the liberty to explore others. The Production Division plans the laboratory for production of vaccines, without intervention of the "specialized" firms that do not have real experience in the production of biological products.
We have been sought by Brazilian and foreign companies to open doors to public-private partnerships. This would transform Butantan into a development center that the company does not have, nor in which it is interested in investing, even if subsidized by the government. The state pharmaceutical industry behaves like the private sector, in that it does not invest in development and innovation. For its part, if Butantan dedicated itself solely to development, without production, it would never improve or even truly test the supposedly developed process. Even once production is installed, Butantan's legal and financial structure allows, given certain criteria, periodic introduction of technological modifications that improve the product or production, which does not take place in a private sector company, which maintains a plant and process while it obtains from it products that have a market, creating profits without new investments. Many innovations in the pharmaceutical industry, as has been denounced in dozens of books, are simply minor and cosmetic alterations in a medication that do not provide the new product greater advantages and benefits than the traditional one.
Limited by the capacity of our small staff, we sought alternatives. We are not in the market for closed technologies and we are associating ourselves with large institutes such as the National Institute of Health (NIH), transforming the knowledge generated into technology and production. We began with the pentavalent rotavirus vaccine, opening the route for other producers in India and China, which along with us reached will reach nearly 50% of the world's population of children in each location with the frequent serotypes. PATH, with resources from the Bill and Melinda Gates Foundation, is assisting the producers of this vaccine, which would cover the prevalent virus in each region and would reduce the cost of the vaccine by about half of the cost of the monovalent vaccine that was imported to Brazil. The agreement reached three years ago to produce rotavirus followed another for the quadrivalent dengue vaccine, and the Pediatric Dengue Vaccine Initiative, once again with resources from the Gates Foundation, is giving priority to assisting Butantan. In the two cases, the NIH has concrete evidence that the vaccines are safe and probably effective. The Butantan-NIH partnership allows completing the technological development, testing the vaccine and producing for Brazil and some other developing countries. In this model in progress, the partnership is basically public (Butantan)-public (NIH). In other countries where there are no longer public institutes such as Butantan, the agreements are with private companies.
Other partnerships include one with Harvard University, with support from PATH, to develop a cellular vaccine against pneumococcos. This vaccine is the result of an internal project at Butantan, which should give origin to a vaccine that would cost some US$ 1-2 per dose, instead of the US$53 of the conjugated 7-valent vaccine. In conjunction with the Sabin Institute-George Washington University, we are contributing to the development of a vaccine against worms (Necator and Schistosoma). Two months do not pass without another institution coming to Butantan to discuss a new project. The same has occurred with Brazilian pharmaceutical companies, with which we are negotiating partnerships. These contacts presuppose that Butantan comes to produce vaccines and other biological products, offering them at reduced cost to the Ministry of Health, which serves the government market, and to private companies that supply the open market. In this way, it is Butantan that assumes the risk of capital investment. This is the agreement for the botulinic toxin type A, which in the public sector is used for certain spastic diseases, and in the private sector mostly for aesthetic purposes. Another type of public-private association began to arise where private companies sought Butantan to develop technology for the private company. In this model, Butantan conducts the development, which implies the construction or use of a pilot plant with Good Manufacturing Practices (GMP). The product can then be clinically tested and the technology transferred to the private company, which will pay royalties to Butantan.
Despite some of the agreements reached, the companies react immediately
to the potential market and profit. They know that it is impossible to compete with products imported in bulk, for either the private or even the government-owned companies. This is the case of Butantan's projects to produce erythropoietin and
alpha interferon with a pharmaceutical company, a market that disappeared when Farmanguinhos began to import these products. Other private companies import the bulk product manufactured in China at very low prices, which makes competition unviable with a better quality product manufactured in Brazil.
The research-development production facilities provides Butantan return on its investments and make it less dependent on funding, and also allows true technological development. The interruption of this link, in which the private industry and even the government see Butantan as a development center that fills the deficiency of private industry, which assumes long term investments and risks, would lead rapidly to the deterioration of what the Institute represents to the country and society. We cannot convert ourselves simply into a supplier of what the private company wants. To not be killed by predatory actions, which they assume are the responsibility of the government to business, we must find a form of symbiosis that contributes to the benefit of society.
Surfactant: the pre-vaccinal-hemoderivatives solution
The potential exists to completely eliminate some viral diseases from the planet. Until now, the only successfully concluded case is smallpox, which is now totally extinct. It appears that polio, which has been eliminated from the Americas, may soon be extinct. Nevertheless, countries with a deficient public health structure or even those that produce and export the vaccine, but do not use it in sufficient quantity, allow polio to survive and be transferred by travelers to other countries, which obliges those countries where polio has been eradicated to continue vaccinations. Rubella was nearly eliminated from the Americas, but wound up reappearing after arriving with travelers. Even with safe and effective vaccines, diseases caused by viruses or bacteria that survive in animals or even live freely can be controlled, but not become extinct.
With the introduction of universal vaccination campaigns in Brazil, child mortality has been reduced and in 2006 was 25/1,000 live births. In São Paulo State it fell to 13/1,000, which is still much higher than countries such as Sweden and Cuba. Half of the mortality represents neonatal mortality, prior to the application of the vaccine. Vaccines that can be administered in the maternity ward, such as BCG (for tuberculosis) and those for hepatitis B, can reduce mortality in the first months of life. Other diseases, such as whopping cough, are frequent and deadly before the fourth month of life, requiring vaccination with DTP in the second and fourth month. Considerable reduction of whopping cough, deadly for newborns, should occur with the application of the recombinant BCG for the S1 subunit of the pertussis toxin.
Nearly 50% of neonatal mortality is caused by pulmonary immaturity, which impedes the opening of the alveolas with a child's first cry. The immaturity is caused by a lack of surfactant, a complex lipoprotein, which can be substituted by one isolated from pig or cow lungs. In less than 15 minutes, babies with this condition can suffocate to death, so the surfactant needs to be available in the delivery room. The cost of the imported surfactant is some R$ 300-500 per dose. Thus the supply of nearly 100 thousand doses of surfactant per year is economically unviable. Butantan developed the production of the surfactant by an original process that allows its production for about R$ 100 per dose, thanks to the recycling of two of the raw materials, the solvent that extracts this surfactant from the surface of the alveolars, and the purification resin, reducing the cost.
This product, already clinically tested, is simply awaiting registration by Brazil's National Sanitary Inspection Agency (Anvisa), and can save 300 babies per day, who now die from suffocation while their mothers watch; and who become pregnant again at the cost of the National Social Security Agency (INSS).
In addition to the phospholipids, the basic surfactant contains two indispensable hydrophobic proteins and a hydrophilic (A) protein, which has a similar structure to the protein of the C1q complement and is also a collectin, which links microorganisms, defending the organism from infection. Its production would be particularly useful for the production of a special surfactant, to which would be added tobramicine, which could be used in the treatment of pneumonia, even that produced by pseudomons common in hospital infections.
According to the Brazilian Constitution, plasma and its byproducts cannot be sold. Since 1993, Butantan has attempted to develop a plant for fractioning human plasma, which was impeded (because it did not have access to the plasma) by what is known as the "vampire mafia", which mediated importation of the hemoderivatives. As an alternative, the plasma was exported for processing. Despite this problem, the idea was maintained to mount a processing center using the Cohn method in which proteins are precipitated with alcohol at low temperatures. This process, which was developed during World War II, continues to be used due to the international monopoly that does not seek to inovate and substitute its plants. Plasma contains about one hundred different proteins. The VIII and IX factors are isolated by cryoprepicitation because they are poorly soluble at low temperatures, but their recovery does not reach 50%, which opened the market for recombinants, giving insufficient attention to the natural factors, which can now be isolated free of virus, without the expense for acquisition of more than 75% of the recombinant factors. The plasma without the factors is precipitated with variable concentrations of alcohol, at different pH and temperature, to obtain albumin (for which there is now limited demand) and the immunoglobulins. In the Cohn method, the VIII and IX factors represent 37% of the value of the plasma and immunoglobulin products 46%. Finally, Butantan began the construction of its plasma processing plant using only chromatography, making it the most modern plant existing. Without using cryoprecipitation or alcohol, it will be possible to recover a higher number of proteins with medical application. One of them is alpha-1 antitripsin, which inhibits the elastase liberated by the neutrophiles that accumulate during the pulmonary infection processes, and which were the cause of death during the flu pandemic of 1918, because they suffocated the victims. The alpha-1 antripsin can be associated to the surfactant in the treatment of pneumonia.
Genome and more vaccines
The Biotechnology Center participated in mapping the genome of the Xillela, which opened new perspectives for the entrance of Butantan into molecular biology. Complete genomes of Schistosoma and of Leptospira were important contributions of the Center's Molecular Biology group, which has the potential to indicate proteins that could be transformed into vaccines. We abandoned, as we mentioned above, the production of erythropoieti and interferon- γ, due to the invasion of imported products. We will invest once again in the porcine VIII factor as a low cost solution for grave hemophiliacs who are resistant to the natural or recombinant VIII factor. We continue to invest, even without the desired intensity, in the vaccines for HPV whose cost would practically absorb all the resources of the Ministry of Health.
We live in a country with great potential to become advanced if we concentrate on our priorities and maintain, in equilibrium with scientific research, a responsible pragmatic attitude. This is what guides the immunobiological program at Butantan.
Received on 9.8.2008 and accepted on 9.12.2008.
Isaias Raw is Professor Emeritus at the University of São Paulo (USP) Medical School and is president of the Fundação Butantan. Winner of the Wessel Award 2005. @ iraw@butantan.gov.br
Hisako G. Higashi is the Director of the Bioindustrial Division of the Instituto Butantan. Winner of the Wessel Award 2007. @ hisa@butantan.gov.br
Publication Dates
-
Publication in this collection
16 Nov 2009 -
Date of issue
Dec 2008