Abstracts
Phylogenetic relationships within the liolaemid lizard genus Phymaturus were studied using parsimony analysis of morphological data. The data set includes 133 characters: 28 described in the literature as apomorphies of the three genera of Liolaemidae (Ctenoblepharys, Liolaemus, and Phymaturus), 21 published characters of allozymes and karyology, 53 characters taken from external morphology across all terminals of Phymaturus, and 31 from the skeletal anatomy. This data set includes representatives of 10 of the 12 species currently recognized in the literature plus twelve other terminals considered in this study and representing independent lineages assigned to patagonicus or palluma. Four of these terminals are described in the present study as new species, one belonging to the palluma group and the other three to the patagonicus group. We performed four analyses using different methods of coding binary polymorphic characters, and a new method for treating continuous characters. The traditional division of the genus in two groups is not supported here, with the patagonicus group resulting paraphyletic in some of the analyses. The palluma group is monophyletic and supported by many characters. A majority rule consensus tree of all runs recovers a reasonably well-resolved topology of the group. All analyses recovered a northern subclade within the palluma group, formed by species distributed in Argentina from northern of San Juan province (north to 30 degrees of latitude). In this analysis palluma from El Planchón (Chile) was found to be more closely related to this northern subclade than any other "palluma" form.
Liolaemidae; Phymaturus; new species; morphology; phylogenetic analysis
Estudaram-se as relações dentro do gênero Phymaturus da familia Liolaemidae, usando análise de parsimônia de uma matriz formada principalmente por dados morfológicos. A matriz inclui 133 caracteres: 28 descritos na literatura como apomorfias dos três gêneros de Liolaemidae (Ctenoblepharys, Liolaemus e Phymaturus), 21 caracteres de alozimas e cariologia, 53 caracteres de morfologia externa e 31 do esqueleto, de todos os terminais de Phymaturus. A matriz inclui representantes de dez das doze espécies reconhecidas na literatura, e outros 12 terminais que neste estudo se consideram linhagens independentes e identificadas como P. patagonicus ou P. palluma na literatura prévia e nas coleções. Quatro destes terminais são descritos neste trabalho como espécies novas, uma pertencente ao grupo de P. palluma e outras três ao grupo de P. patagonicus. Realizaram-se quatro análises usando quatro métodos diferentes para codificar binariamente caracteres polimórficos, e um novo método para codificar os caracteres contínuos. A divisão tradicional do gênero em dois grupos não é apoiada pelo presente estudo, o grupo de P. patagonicus é parafilético em parte da análise. O grupo de P. palluma é monofilético e se sustenta por vários caracteres, as árvores de consenso por maioria, de todas as análises, mostraram com exceção de um par de politomías, uma topologia do grupo bem resolvida. Dentro do grupo de P. palluma, encontra-se um subclado formado por espécies distribuídas no norte da Argentina desde o norte da província de San Juan (ao norte dos 30° de latitude). Nesta análise, o P. palluma de El Planchón (Chile), relaciona-se mais com este subclado do norte que com qualquer outra forma de "P. palluma".
Liolaemidae; Phymaturus; novas espécies; morfologia; análise filogenética
A morphology-based phylogeny of Phymaturus (Iguania: Liolaemidae) with the description of four new species from Argentina
Fernando LoboI, II; Sebastián QuinterosI
IFacultad de Ciencias Naturales, Universidad Nacional de Salta-CONICET. Avda. Bolivia 5150. 4400 Salta. Argentina
IIE-mail: flobo@unsa.edu.ar
ABSTRACT
Phylogenetic relationships within the liolaemid lizard genus Phymaturus were studied using parsimony analysis of morphological data. The data set includes 133 characters: 28 described in the literature as apomorphies of the three genera of Liolaemidae (Ctenoblepharys, Liolaemus, and Phymaturus), 21 published characters of allozymes and karyology, 53 characters taken from external morphology across all terminals of Phymaturus, and 31 from the skeletal anatomy. This data set includes representatives of 10 of the 12 species currently recognized in the literature plus twelve other terminals considered in this study and representing independent lineages assigned to patagonicus or palluma. Four of these terminals are described in the present study as new species, one belonging to the palluma group and the other three to the patagonicus group. We performed four analyses using different methods of coding binary polymorphic characters, and a new method for treating continuous characters. The traditional division of the genus in two groups is not supported here, with the patagonicus group resulting paraphyletic in some of the analyses. The palluma group is monophyletic and supported by many characters. A majority rule consensus tree of all runs recovers a reasonably well-resolved topology of the group. All analyses recovered a northern subclade within the palluma group, formed by species distributed in Argentina from northern of San Juan province (north to 30 degrees of latitude). In this analysis palluma from El Planchón (Chile) was found to be more closely related to this northern subclade than any other "palluma" form.
Keywords: Liolaemidae; Phymaturus; new species; morphology; phylogenetic analysis.
RESUMO
Estudaram-se as relações dentro do gênero Phymaturus da familia Liolaemidae, usando análise de parsimônia de uma matriz formada principalmente por dados morfológicos. A matriz inclui 133 caracteres: 28 descritos na literatura como apomorfias dos três gêneros de Liolaemidae (Ctenoblepharys, Liolaemus e Phymaturus), 21 caracteres de alozimas e cariologia, 53 caracteres de morfologia externa e 31 do esqueleto, de todos os terminais de Phymaturus. A matriz inclui representantes de dez das doze espécies reconhecidas na literatura, e outros 12 terminais que neste estudo se consideram linhagens independentes e identificadas como P. patagonicus ou P. palluma na literatura prévia e nas coleções. Quatro destes terminais são descritos neste trabalho como espécies novas, uma pertencente ao grupo de P. palluma e outras três ao grupo de P. patagonicus. Realizaram-se quatro análises usando quatro métodos diferentes para codificar binariamente caracteres polimórficos, e um novo método para codificar os caracteres contínuos. A divisão tradicional do gênero em dois grupos não é apoiada pelo presente estudo, o grupo de P. patagonicus é parafilético em parte da análise. O grupo de P. palluma é monofilético e se sustenta por vários caracteres, as árvores de consenso por maioria, de todas as análises, mostraram com exceção de um par de politomías, uma topologia do grupo bem resolvida. Dentro do grupo de P. palluma, encontra-se um subclado formado por espécies distribuídas no norte da Argentina desde o norte da província de San Juan (ao norte dos 30° de latitude). Nesta análise, o P. palluma de El Planchón (Chile), relaciona-se mais com este subclado do norte que com qualquer outra forma de "P. palluma".
Palavras-chave: Liolaemidae; Phymaturus; novas espécies; morfologia; análise filogenética.
INTRODUCTION
Phymaturus comprises a group of iguanian lizards inhabiting rocky places of Patagonia and both eastern and western mid- to high-elevation slopes of the Andes. This group of viviparous and herbivorous lizards reaches its northern limit in the Puna region of Catamarca in northern Argentina. The southernmost-distributed species is indistinctus, living at the latitude of 45°30' South. According to Etheridge (1995) species of this clade of iguanians are characterized by having wide and flattened head and body, tail with a regular whorls of spinose scales, lateral nuchal skin folds with fat-filled pouches, a short interclaviculae, among other characters that are exclusive and provide strong evidence of monophyly.
At the time of Donoso Barros (1964) and Peters and Donoso Barros (1970), the genus included only one species with two subspecies: palluma palluma (Molina) 1782, and palluma patagonicus (Koslowsky) 1898). Most taxa recognized as species today were described during the 1970s80s: P. patagonicus payunae, P. p. somuncurensis, P. p. zapalensis, P. p. indistinctus, and P. p. nevadoi by Cei & Castro (1973); P. mallimaccii by Cei (1980); P. punae by Cei et al., (1983); and P. antofagastensis by Pereyra (1985). Despite publication of several systematic studies on the genus over the last 30 years (Cei & Lescure, 1985; Lescure & Cei, 1991; Pereyra, 1992a; among others), the phylogenetic relationships among Phymaturus species remain enigmatic. Pereyra (1992a) studied the external morphology, karyology, and allozymes of six species/populations of Phymaturus, but his study applied phenetic techniques (cluster analysis), which established phenetic distances among taxa but did not provide a genealogical reconstruction. Much of Pereyra's (1992a) information was included and reevaluated in the present study. Etheridge (1995) provided the first extensive study of genera now included in the Liolaemidae (Phymaturus, Liolaemus, and Ctenoblepharys), and proposed a cladistic taxonomy based on morphological characters. Within Phymaturus Etheridge (1995) recognized two groups of species, the palluma group with four species, and the patagonicus group formed by six species (formerly considered subspecies of patagonicus by Cei [1986]). Characters defining the palluma group were the non-imbricate superciliaries, five or more suboculars, three to four rows of lorilabials, mental narrower than rostral and usually in contact with sublabials, caudal spines well developed, and two annuli per segment. For the patagonicus group, Etheridge (1995) identified a splenial short and a fused Meckel's groove. However, Etheridge (1995) noted that these groups may not necessarily be monophyletic. Following this work, no additional studies were published that sought to recover the genealogical relationships within the genus, yet, many questions remain, like the validity and or monophyly of Etheridge's (1995) groups of species, and the phylogenetic relationships among species. Recently, Cei & Videla (2003) and Scolaro & Cei (2003) described two new species for the genus and discussed the unresolved problem of the identity of the true palluma, a lizard collected by Charles Darwin on his voyage on the Beagle and posteriorly described by Bell (1843). The latter issue remains a problem that needs to be resolved through the examination of populations on both sides of the Andes, and compared to the type material deposited at the British Museum of Natural History.
The goal of this study is to provide a first comprehensive analysis of Phymaturus, using cladistic methods. We studied the external morphology and skeletons of an extensive sample of terminals and individuals. We examined and described more than 100 informative characters, most of them used in the systematics of Phymaturus for the first time, as well as other characters taken from the literature.
MATERIALS AND METHODS
For the phylogenetic analysis we were able to reexamine the type series of nine of the 12 currently recognized species (with the exception of palluma, verdugo, and calcogaster). Characters used in this study were obtained primarily from the skeleton, squamation, morphometry, and body patterns. The allozyme data set plus two chromosomal characters of Pereyra (1992a) were also included. Skeletal characters were visualized from cleared and stained material following Wassersug's (1976) technique, which allows differential staining of cartilage and bone. For some specimens (MVZ and SDSU dry skeletons) however, cartilaginous structures (larynx, trachea, and hyoid apparatus) were not available because they were prepared as dry skeletons. External characters were scored from formalin-preserved specimens stored in alcohol 70%. Characters for phylogenetic analysis were scored only from adults. Ontogenetic shift in external morphology between juveniles and adults was recorded and it is presented in a separated section. Scale terminology follows a previous study on the Liolaemus chiliensis group (Lobo, 2001), measurements were taken with digital calipers (± 0.01 mm) and following the definitions of Laurent (1984). Skeleton terminology follows previous studies (Lobo & Abdala, 2001, 2002). Museum numbers and localities for specimens included in this study are listed in Appendix I APPENDIX I . We selected characters based on previous cladistic studies in Liolaemus (Lobo, 2001; Lobo & Abdala, 2001, 2002), or those described for Phymaturus (Cei, 1980, 1986, 1993; Cei & Castro, 1973); Cei et al., 1983; Etheridge, 1995), and we reevaluated many of characters considered informative for the genus by Pereyra (1992a) (among meristic, allozyme, and karyological characters he studied). For a details and a list of characters see Appendix II APPENDIX II . He found characters discriminating payunae and cf. palluma (CH) from the rest of species (morphometric characters in page 71, Table 18), head length, number of scales on the dorsum of the head (equivalent to his LCI), and head width; from his meristic characters that he found useful for discriminating payunae and mallimaccii from other species (page 68, Table 17) we used number of scales in contact to mental, scales around midbody, dorsals along the back included in a head length, and number of suboculars. Also we included his data set of allozymes (19 characters) and two from karyology (diploid chromosomal number and number of microchromosomes in females), which were available for four of our terminals (P. antofagastensis, P. mallimaccii, P. palluma from Mendoza, and P. payunae). We added diploid chromosomal number to two other terminals, P. dorsimaculatus (from Copahue, Neuquén Province) and P. excelsus (from Ojo de Agua, Rio Negro) following Morando et al., (2001).
Phylogenetic analyses were conducted with parsimony software (TNT: Tree Analysis Using New Technology, vers. 1.0; Goloboff et al., (2003). We choose TNT for our analysis because this is the only program that allows the analysis of continuous characters without first converting them in discrete characters (as proposed by Thiele, 1993). Data matrices are available at www.unsa.edu.ar/acunsa. There is more than one matrix because different coding methods were applied for binary polymorphic characters. The first block in matrix is comprised of continuous characters (26 for external morphology and 14 for skeletal anatomy), the same characters are included in the following block (characters 88106; 109113; 135148). During searches we deactivate these characters if we use the range method for continuous, and the opposite to apply Thiele's gap-weighting method (Thiele, 1993). For TNT numeration of characters begins with the first block (with character "0" designating the first one), so the second block (traditional format) starts with character 40. The list of characters in text 0132 are equivalent in TNT format of our matrices to 40172. In our analysis we considered the following TNT characters non-additive 6886 (allozyme characters: 2846); 128 and 130 (throat pattern of males and females, dorsal pattern of tails: 8890), 168 and 170 (128 and 130, "smooth pattern" and dorsal pattern black with two rows of occelli); all remaining characters were additive. Binary polymorphic characters were analyzed in four different ways: any-instance (the polymorphic species is considered already having the novelty, so it is coded "1" as in species that this state is fixed), scaled (polymorphic species have an intermediate state "1" in an ordered series between species not having that derived state "0" and those having the derived state "2"), frequency bins (frequency of the presence of the derived state is used to score each species, percentage ranges are divided by 09 states for TNT analysis), and missing (question mark for polymorphic species). For more details on these methods and performance of them in cladistic analysis see Wiens (2000) TNT uses Farris'optimization (Farris, 1970) to estimate distances and costs among ranges, when ranges between two terminals overlap, TNT assumes zero cost. In this study we entered ranges for continuous data to the program considering a mean ± SD as a range for any continuous character, and we standardized all continuous characters dividing the ranges by the major data and multiplying this by ten. In this way the costs of the continous characters are similar to the others.
For frequency bins we preferred not dividing by more states than 10 because the limited sample size we had of some species.
For rooting our trees we included the other two liolaemid genera, Liolaemus (three species: L. kingii, L. tenuis, and L. pseudoanomalus) and the monotypic Ctenoblepharys. Relationships among genera in Liolaemidae were once controversial (Frost & Etheridge, 1989; Etheridge, 1995), yet according to the last revision of intergeneric relationships (Etheridge, 1995), there are many synapomorphies supporting the monophyly of each of the three genera. Recent DNA analyses support a Phymaturus-Liolaemus sister-taxon relationship with Ctenoblepharys adspersa as the basal member of the family (Schulte et al., 2003; Espinoza et al., 2004).
RESULTS
1) Choice of terminal taxa
We included in the present study all recognized species of Phymaturus in literature (Etheridge & Espinoza, 2000) except the recently described P. verdugo Cei & Videla, 2003 and P. calcogaster Scolaro & Cei, 2003: cf. palluma, antofagastensis, mallimaccii, punae, patagonicus, nevadoi, payunae, zapalensis, indistinctus, and somuncurensis. Also we included spurcus, a recently revalidated species (Lobo & Quinteros, in press). In addition to these species, we included twelve populations that are assigned to named species yet show morphological evidence of evolutionary isolation (likely representing independent lineages) from nominal forms. Four of these terminals are described below as new species. We follow Cei & Videla (2003) in recognizing that the issue of the provenance of P. palluma cannot be readily resolved (i.e., populations inhabitating the eastern and western slopes of the Andes exhibit morphological [this study], karyological [Pereyra, 1992a], and DNA [R. Espinoza, unpubl. data] differences), so we use cf. palluma, rather than P. palluma in this study.
I Description of four new species of Phymaturus
Of the twelve terminals added to this analysis to the currently recognized species, four exhibit obvious discriminating characters (squamation and patterns) that justitfy their description as new species.
Phymaturus dorsimaculatus sp. nov.
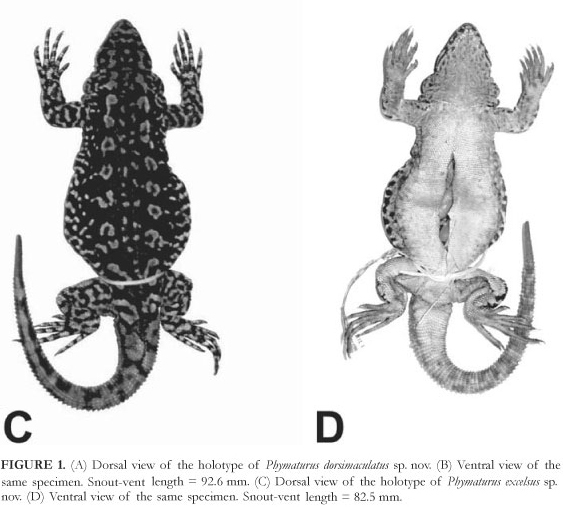
Holotype (Figure 1A and B): MCN 1573. Copahue, Dpto. Ñorquin. 37°49'S; 71°06'W. Neuquén, Argentina. Abdala, C.; Avila, L.; F. Lobo, & M. Morando, collectors. 13 January 1999.
Paratypes: MCN 157172, 157475. Same data as holotype. MCN 156869. Termas de Copahue, Dpto. Ñorquin, Neuquén, Argentina. 37°49'14"S; 71°05'12"W; 2050 m. 13 January 1999. MCN 156667. Copahue, Dpto. Ñorquin, Neuquén.
Diagnosis: Phymaturus dorsimaculatus belongs to the palluma group (sensu Etheridge, 1995) because it has square-shaped non-imbricate superciliaries, rugose dorsal scales of the tail, usually a fragmented subocular, and the subocular-supralabials separation is two or more scale rows. It is distinguishable from all other species in the group by its unusual dorsal pattern. Dorsal pattern from occiput to the posterior region of trunk with black transverse bands interrupted medially (Figure 1A). Phymaturus verdugo is a larger species (106-120 mm both sexes; Cei & Videla, 2003) than dorsimaculatus (76.1-92.6), has a different dorsal pattern, and a patternless tail (ringed in dorsimaculatus). This new species has a different karyotype number (2n = 36) versus 2n = 26 of P. verdugo (Morando et al., 2001; Cei & Videla, 2003). Adults of cf. palluma (PA) lack melanism on head and neck and all other members of the palluma group have patternless tails. Phymaturus dorsimaculatus never have a divided rostral scale as does many punae, antofagastensis, cf. antofagastensis SC (Sierra de Calalaste and Cuesta de Randolfo), and punae LR (La Rioja). Most specimens from northern Argentina of the palluma group exhibit a homogeneous and dense "spray" pattern on their dorsum (Figure 12C) and lack black reticulation or spots.
Description of holotype: Female. Snout-vent length (SVL) 92.6 mm. Head length 16.2 mm. Head width 16.8 mm. Head height (at parietal) 8.8 mm. Axilla-groin 46.7 mm (50.4% of SVL). Tail length (complete, not regenerated) 83.3 mm (0.9 times SVL). Body moderately wide, trunk width: 40.2 mm (43.4% of SVL). Eighteen dorsal head scales. Dorsal head scales smooth, with scale organs more abundant in prefrontal region. Six, five, five, and four scale organs in each postrostral. Nasal scale not in contact with rostral, bordered by nine scales. Canthal separated from nasal by one scale. Loreal region flat. Seven enlarged supralabial scales with seventh upturned posteriorly, contacting subocular. Nine enlarged infralabials. Auditory meatus oval; auricular scale absent, three to four projecting scales on anterior margin of auditory meatus (both sides). Nine convex, juxtaposed, smooth temporals. Rostral undivided. Mental subpentagonal, in contact with six scales. Interparietal bordered by five scales, parietals of similar size. Frontal region without an azygous scale. Supraorbital semicircles incomplete posteriorly on both sides. No distinctly enlarged supraoculars. Twelve non imbricate subquadrangular supercilliaries. Sixteen upper and thirteen lower ciliaries (right side). Subocular fragmented in two (left side) and three scales (right side), separated from supralabials by three to one row of lorilabials. Ten lorilabials. Preocular separated from lorilabial row by three scales. Postocular equal in length to preocular. Chinshields not enlarged (similar size of that of sublabials) forming a longitudinal row of five scales. Scales of throat round, flat, and juxtaposed. Seventy-four gulars between auditory meatus. Lateral nuchal folds well developed, with granular scales over longitudinal fold that are smaller than dorsals. Antehumeral pocket well developed. Fifty-six scales between auditory meatus and shoulder. In ventral view gular fold not well developed and posterior gular folds present with their anterior margins lacking enlarged scales on their borders. Dorsal scales round, smooth, juxtaposed. Thirty dorsal scales along midline of the trunk in a distance equivalent to head length. Scales around midbody 210. Mid-dorsal scales slightly enlarged, becoming smaller and granular on flanks and toward belly. Ventral scales larger than dorsals; 172. Between mental and precloacal area. No precloacal pores. Brachial and antebrachial scales smooth with round posterior margins. Supracarpals laminar, round, smooth. Subdigital lamellae of fingers with five keels (more conspicuous in proximal lamellae). Number of subdigital lamellae of fingers I: 9; II: 13; III: 17; IV: 19; V: 12. Claws moderately long. Supradigital lamellae convex, smooth, imbricate. Infracarpals and infratarsals with round margins and two to three obtuse keels. Supracarpals and supratarsals smooth, with round posterior margins. Subdigital lamellae of toes I: 8; II: 13; III: 20; IV: 23; V: 15.
Variation: Based on 15 specimens (4 females, 7 males, 2 juvenile females, and 2 juvenile males). Snout-vent length 62.292.6 mm (x = 79.3; SD = 9.4). Head length 0.170.22% (x = 0.20; SD = 0.01) of SVL. Tail length 0.811.04 (x = 10.95; SD = 0.07) times SVL. Scales around midbody 200256 (x = 229.55; SD = 17.4). Dorsal head scales 1724 (x = 19.4; SD = 1.7). Ventrals 160199 (x = 176.5; SD = 11.3). Precloacal pores in males 510 (x = 8.3; SD = 2.1). Scales surrounding interparietal 58 (x = 6.5; SD = 0.9). Scales of neck along longitudinal fold from posterior border of auditory meatus to shoulder 6287 (x = 76.6; SD = 9.1). Gulars 5586 (x = 78.4; SD = 8.8). Scales between rostral and frontal 712 (x = 9.3; SD = 1.6). Subdigital lamellae of fourth toe 2024 (x = 22.5; SD = 1.5). Dorsal pattern black-forked is typical in adult females and all juveniles, but less conspicuous or restricted to the region of the neck and shoulder in adult males. One male have completely melanic head.
Color of holotype in alcohol (Figure 1A and B): Dorsal background color brown with a distinct pattern in black extended from occiput to the posterior region of trunk forming transverse bands that are interrupted medially. Finer irregular black reticulation distributed along the vertebral region reaching the base of the tail. The tail is ringed with darker brown bands two scales wide. Forearms and hindlimbs with fine reticulation. Head not melanic. Throat gray-light brown with reticulate brown more evident below jaws. Chest black. Abdominal region variegated.
Color in life: Not available.
Etymology: The epithet refers to the conspicuous pattern of black spots over the neck, shoulders, and dorsum of the trunk of this new species. It is a compound name (originated from two latin words dorsum that means back and macula that means spot, mark).
Distribution: Only known from the type locality, Copahue, Neuquén province, Argentina, 37°49'14"S, 21°05'12"W; 2050 m elevation.
Phymaturus excelsus sp. nov.
Holotype: MCN 1582. Ruta prov. 6, 1 km NW of Ojo de Agua, Dpto. Ñorquinco, Rio Negro, Argentina. L. Avila & M. Morando, collectors. 41°32'30"S; 69°51'33"W; 1141 m.
Paratypes: MCN 1386, 1388. Ojo de Agua. Ruta 6. Dpto. Ñorquinco, Rio Negro, Argentina. Abdala, C.; F. Lobo; I. Martínez Oliver; S. Quinteros. MCN 922 (CS), 15831586. Same data as holotype. MCN 158788. No data.
Diagnosis: Phymaturus excelsus belongs to the patagonicus group (sensu Etheridge, 1995) because it has flat imbricate superciliaries, non-rugose dorsal scales on tail, subocular usually not fragmented, and subocular-supralabials separated by one scale row. This new species differs from all other members of this group in its unique dorsal pattern, with a dorsal background in black and a pair of longitudinal series of white occelli (fig. 1C). Similar patterns with a paired series of occelli are found (but in different colors, shapes, and arrengements) in payunae, zapalensis, and spectabilis sp. nov. (described below). Phymaturus payunae and P. zapalensis are sexually dimorphic in dorsal patterns, whereas in excelsus there are no pattern differences between the sexes. Dominant colors in excelsus are black and white, whereas in spectabilis are brown and light brown, and occelli in this latter species are much wider and more symmetrical.
Description of holotype: Male. SVL 82.5 mm. Head length 16.0 mm. Head width 14.2 mm. Head height (at parietal) 9.0 mm. Axilla-groin 41.9 mm (50.8% of SVL). Tail length (complete, not regenerated) 82.8 mm (1.00 times SVL). Trunk width: 35.6 mm (43.1% of SVL). Twenty one smooth dorsal head scales. Five, five, and four scale organs in each postrostral. Nasal scale not in contact with rostral (separated by one scale), bordered by eight scales. Canthal separated from nasal by two scales. Loreal region flat. Nine enlarged supralabial scales with the seventh upturned posteriorly, not contacting subocular (separated by one lorilabial). Seven enlarged infralabials. Auditory meatus oval; auricular scale absent, three to four projecting scales on anterior margin of auditory meatus (both sides). Eleven convex, juxtaposed, smooth temporals. Rostral undivided. Mental subpentagonal, in contact with four scales. Interparietal bordered by seven scales, parietals smaller. Frontal region without an azygous scale. Supraorbital semicircles incomplete posteriorly on both sides. No distinctly enlarged supraoculars. Nine distinctly imbricate superciliaries. Thirteen upper and twelve lower ciliaries (right side). Subocular elongate, longer than eye diameter, separated from supralabials by a single row of lorilabials. Eleven lorilabials; tenth through eleventh contacting subocular. Preocular separated from lorilabial row by one scale. Chinshields forming a longitudinal row of seven or eight enlarged scales. Scales of throat round, flat, and juxtaposed. Seventy gulars between auditory meatus. Lateral nuchal folds well developed with granular scales over longitudinal fold that are smaller than dorsals. Antehumeral pocket well developed. Sixty-eight scales between auditory meatus and shoulder. In ventral view, gular fold absent, and posterior gular folds present with their anterior margins bordered by enlarged scales. Dorsal scales round, smooth, juxtaposed. Forty-three dorsal scales along midline of the trunk in a distance equivalent to head length. Scales around midbody 202. Middorsal scales same size of those on flanks. Ventral scales larger than dorsals. Ventral scales between mental and precloacal pores 182. Eleven precloacal pores forming an interrupted row. Brachial and antebrachial scales smooth with round posterior margins. Supracarpals round and smooth. Subdigital lamellae of fingers with five to three keels, in number I: 11; II: 17; III: 24; IV: 25; V: 17. Claws moderately long. Supradigital lamellae convex and imbricate. Infracarpals and infratarsals trifid with round margins. Supracarpals and supratarsals smooth with round posterior margins. Subdigital lamellae of toes with three to five keels: I: 12; II: 19; III: 24; IV: 29; V: 19.
Variation: Based on 8 adult specimens (4 females and 4 males). SVL 77.089.7 mm (x = 85.9; SD = 4.2). Head length 0.170.19% (x = 0.18; SD = 0.01) of SVL. Tail length 1.01.07 (x = 1.02; SD = 0.03) times SVL. Scales around midbody 178223 (x = 201.9; SD = 14.0). Dorsal head scales 1722 (x = 20.13; SD = 1.55). Ventrals 156182 (x = 168.25; SD = 8.4). Precloacal pores in males 911 (x = 10; SD = 1.0). Scales surrounding interparietal 68 (x = 7.0; SD = 0.8). Scales of neck along longitudinal fold from posterior border of auditory meatus to shoulder 6292 (x = 71.1; SD = 10.4). Gulars 6088 (x = 71.5; SD = 8.9). Scales between rostral and frontal 79 (x = 8.4; SD = 0.7). Subdigital lamellae of fourth toe 2628 (x = 27.0; SD = 0.6).
Color of holotype in alcohol (Figure 1C and D): Dorsal background black on trunk, shoulder, neck, and head. A paired series of eight lateral white or cream occelli are conspicuous from the occiput to the thighs. Between these two rows of occelli are irregularly located small light-cream spots. Dorsum of limbs and tail variegated. Ventral coloration ligth gray to white, inmaculate exhibiting small spots on the throat.
Color in life: see Figure 3. Dorsal background of trunk, head and limbs black, with light brown ocelli over the dorsum. A reticulate pattern in black and light brown over the head, limbs and dorsal surface of tail.
Etymology: Phymaturus excelsus is Latin for "distinguished," which describes the peculiar and distinct pattern exhibited by these lizards.
Distribution (Figure 5): Phymaturus excelsus is known only from its type locality, where it lives syntopically with Phymaturus spurcus a species described by Barbour (1921) from Estancia Huanuluan, 40 km to the north (straight line).
Phymaturus spectabilis sp. nov.
Holotype: MCN 1203. 28 km south of Ingeniero Jacobacci, Rio Negro province, Argentine (on Provintial Road 6). C. Abdala, F. Lobo, I. Martínez Oliver, and S. Quinteros, collectors.
Paratypes: MCN 12041215. Same data as holotype.
Diagnosis: Phymaturus spectabilis belongs to the patagonicus group (sensu Etheridge, 1995) because it has flat imbricate superciliaries, non-rugose dorsal scales of the tail, subocular unique usually not fragmented, and subocular-supralabials separation given for one row of scales. It is distinguishible of all other species of the genus by its unusual dorsal pattern (Figures 2 and 4).Phenetically the species more close to P. spectabilis is P. excelsus. The last one exhibit a general dorsal pattern black with smaller and more occelli than spectabilis (78 among shoulder and the level of thighs versus 56) and several markings irregularly distributed on its vertebral field between the series of dorsal occelli.
Description of holotype: Female. SVL 95.8 mm. Head length 16.6 mm. Head width 15.1 mm. Head height (at parietal) 8.6 mm. Axilla-groin 53.2 mm (55.5% of SVL). Tail length (complete, not regenerated) 98.9 mm (1.03 times SVL). Body moderately wide, trunk width 42.8 mm (44.7% of SVL). Twenty-two smooth dorsal head scales. Two, two, and four scale organs in each postrostral. Nasal not in contact with rostral, bordered by nine scales. Canthal separated from nasal by two scales. Loreal region flat. Ten enlarged supralabial scales with seventh upturned posteriorly but not contacting subocular. Nine enlarged infralabials. Auditory meatus oval; auricular scale absent, five projecting scales on anterior margin of auditory meatus. Eleven smooth, convex, juxtaposed temporals. Rostral undivided. Mental subpentagonal, in contact with four scales. Interparietal bordered by six scales. Frontal region without an azygous scale. Supraorbital semicircles incomplete posteriorly on both sides. No distinctly enlarged supraoculars. Seven imbricate flat superciliaries. Fifthteen upper ciliaries (right side). Subocular fragmented into two scales, separated from supralabials by one row of lorilabials. Eleven lorilabials, the tenth and eleventh contacting suboculars. Preocular separated from lorilabial row by one scale. Chinshields not enlarged. Scales of throat round, flat, and juxtaposed. Sixty-nine gulars between auditory meatus. Lateral nuchal folds well developed, with granular scales over longitudinal fold that are smaller than dorsals. Antehumeral pocket well developed. Eighty-four scales between auditory meatus and shoulder. In ventral view gular fold not well developed and posterior gular folds present with enlarged scales on their anterior margins. Dorsal scales round, smooth, juxtaposed. Forty-one dorsal scales along midline of the trunk in a distance equivalent to head length. Scales around midbody 224. Middorsal scales not enlarged in comparison to those along flanks. Ventral scales larger than dorsals. Ventral scales between mental and the posterior bordering of the cloaca 169. No precloacal pores. Brachial and antebrachial scales smooth with round posterior margins. Supracarpals laminar, round, smooth. Subdigital lamellae of fingers with 35 keels (more conspicuous in proximal lamellae). Number of subdigital lamellae of fingers I: 12; II: 17; III: 24; IV: 25; V: 17. Claws moderately long. Supradigital lamellae convex, imbricate. Infracarpals and infratarsals with round margins and two to three obtuse keels. Supracarpals and supratarsals smooth, with round posterior margins. Subdigital lamellae of toes I: 13; II: 19; III: 23; IV: 29; V: 21.
Variation: Based on 7 adult specimens (5 females and 2 males). SVL 86.897.5 mm (x = 90.6; SD = 4.3). Head length 0.160.18% (x = 0.17; SD = 0.01) of SVL. Tail length 1.001.15 (x = 1.07; SD = 0.06) times SVL. Scales around midbody 191224 (x = 206.6; SD = 10.3). Dorsal head scales 2022 (x = 21.4; SD = 0.8). Ventrals 145178 (x = 164.8; SD = 10.4). Nine precloacal pores in both males. One female with two precloacal pores. Scales surrounding interparietal 68 (x = 6.7; SD = 0.8). Scales of neck along longitudinal fold from posterior border of auditory meatus to shoulder 7087 (x = 78.0; SD = 5.8). Gulars 6191 (x = 78.4; SD = 10.5). Scales between rostral and frontal 811 (x = 9.4; SD = 0.9). Dorsal pattern of white occelli numbering 78 between shoulder to the level of thighs, otherwise light brown over a black background in all but two adult specimens that lack occelli, yet retain the general brown pattern. Five juveniles also with the same occellated pattern.
Color of holotype in alcohol (Figure 2A and B): Dorsal background predominantly brown and black, six white and brown occelli delimited in black and exhibiting one to two small black spots in the middle of each occellation. Dorsal pattern of tail variegated. Beyond shoulders occelli are fused reaching the parietal region of head. Central region of head black flanked by a pair of brown bands that reach the nasal region anteriorly. Ventral surfaces light gray, lateral margins of abdomen and chest with small black diffuse spots, throat with few very small spots, more conspicuous under jaws. This light gray coloration becomes light brown gradually along the flanks. Ventral surface of tail is light brown variegated with dark brown.
Color in life: See Figure 4. Similar pattern to that described for excelsus, with the light brown fields widely extended (ocelli over dorsum and pattern of head, limbs and dorsal surface tailand).
Etymology: The epithet spectabilis is Latin and means "notable, showy" in reference to the distinct pattern of dorsal occellations in this new species.
Distribution (Figure 5): Only known from the type locality, 28 km south of Ingeniero Jacobacci, Rio Negro, Argentina.
Phymaturus tenebrosus sp. nov.
Holotype: MCN 1271. 20 km south of Cerro Alto, National Road Nº 40, Rio Negro, Argentina. C. Abdala, F. Lobo, I. Martínez Oliver, and S. Quinteros, collectors.
Paratypes: MCN 12641270, 127273. Same data as holotype.
Diagnosis: Phymaturus tenebrosus belongs to the patagonicus group (sensu Etheridge, 1995) because it has flat imbricate superciliaries, non-rugose dorsal scales on tail, subocular usually not fragmented, and subocular-supralabials separated by one scale row. The new species is distinguishible from all other species of the genus by its dorsal pattern: black in most specimens, some with very fine, sparse white spots (e.g., holotype). This melanissm is dark brown in some specimens. The morphologically most similar species to P. tenebrosus are P. zapalensis and the recently described P. calcogaster, from which this new species can be readily distinguished. Phymaturus zapalensis has a more dense pattern of dorsal spots and very often a pattern of reticulation/occellation and obvious sexual dimorphism (females with variegated pattern) and P. calcogaster, which was described solely from the male holotype (Scolaro & Cei, 2003), which has a peculiar pattern not seen in P. tenebrosus: dorsum with larger and homogeneous white spots arranged in trasverse rows, a subocular fragmented into five scales (P. tenebrosus usually lacks subocular fragmentation), and the throat of P. calcogaster is variegated.
Description of holotype Male. SVL 87.2 mm. Head length 16.2 mm. Head width 15.5 mm. Head height (at parietal) 9.2 mm. Axilla-groin 46.5 mm (53.3% of Snout-vent length). Tail length (complete, not regenerated) 81.1 mm (0.93 times SVL). Body moderately wide, trunk width: 33.3 mm (0.38% of SVL). Twenty-two smooth dorsal head scales. Seven, four, and seven scale organs in each postrostral. Nasal bordered by eight scales, not in contact with rostral. Canthal separated from nasal by two scales. Loreal region flat. Eight enlarged supralabial scales with seventh upturned posteriorly but not contacting subocular. Seven enlarged infralabials. Auditory meatus oval with five projecting scales on the anterior margin. Auricular scale absent. Nine convex, juxtaposed temporals. Rostral undivided. Mental subpentagonal, in contact with four scales. Interparietal bordered by seven scales. Frontal region without an azygous scale. Supraorbital semicircles incomplete posteriorly. No distinctly enlarged supraoculars. Nine imbricate flat superciliaries. Subocular unfragmented, separated from supralabials by one row of lorilabials. Nine lorilabials, the eight to ninth contacting subocular. Preocular separated from lorilabial row by two scales. Scales of throat round, flat, and juxtaposed. Eighty-one gulars between auditory meata. Lateral nuchal folds well developed, with granular scales over longitudinal fold. Antehumeral pocket well developed. Eighty-one scales between auditory meatus and shoulder. In ventral view, gular fold not well developed and posterior gular folds present with their anterior margins with enlarged scales on their borders. Dorsal scales round, smooth, juxtaposed. Thirty-nine dorsal scales along midline of the trunk in a distance equivalent to head length. Scales around midbody: 202. Mid-dorsal scales not enlarged in comparison to those on flanks. Ventral scales larger than dorsals. Ventral scales between mental and precloacal pores: 170. Nine precloacal pores. Brachial and antebrachial scales smooth with rounded posterior margins. Supracarpals laminar, round, smooth. Subdigital lamellae of fingers with 35 keels (more conspicuous in proximal lamellae). Number of subdigital lamellae of fingers I: 11; II: 16; III: 22; IV: 24; V: 15. Claws moderately long. Supradigital lamellae convex, imbricate. Infracarpals and infratarsals with round margins and 23 obtuse keels. Supracarpals and supratarsals smooth, with round posterior margins. Subdigital lamellae of toes I: 13; II: 17; III: 18; IV: 23; V: 21.
Variation: Based on 16 adult specimens (12 females and 4 males from both known localities). SVL 85.0107.5 mm (x = 94.8; SD = 6.4). Head length 0.150.19% (x = 0.17; SD = 0.01) of SVL. Tail length 1.001.41 (x = 1.21; SD = 0.18) times SVL. Scales around midbody 171236 (x = 199.2; SD = 20.2). Dorsal head scales 1625 (x = 20.2; SD = 2.18). Ventrals 149185 (x = 171.4; SD = 14.15). Precloacal pores in males 79 (x = 8.5; SD = 1.0). Scales surrounding interparietal 58 (x = 6.9; SD = 0.9). Scales of neck along longitudinal fold from posterior border of auditory meatus to shoulder 5888 (x = 70.9; SD = 8.3). Gulars 65100 (x = 77.8; SD = 9.3). Scales between rostral and frontal 611 (x = 8.7; SD = 1.3). Dorsal and flank pattern in most specimens black, in some specimens with small and scacerly distributed white spots. Some specimens brown morph with conspicuous black spots on flanks (similar to that exhibited by specimens of P. zapalensis). Not all specimens have strong ventral coloration (orange in brown individuals, yet mustard or dark gray in black specimens), suggesting that this coloration may be related to season or physiological conditions.
Color of holotype in alcohol (Figure 2C and D): Dorsal background with black spots and small white markings that are smaller and more densely distributed middorsally. This pattern extends over the head and dorsal surfaces of the limbs. The tail is patternless. General coloration of ventral surfaces (throat, limbs, and tail) gray, with slight yellow color in the posterior region of abdomen continuous over the cloaca and thighs.
Color in life: See Figure 4. Dorsum black scattered with small white spots. Margins of ventral region gray and central areas of chest, abdomen, ventral surfaces of thighs and cloaca mustard. Females can exhibit their bellies light gray to orange.
Etymology: The epithet tenebrosus Latin word that means "dark, gloomy" in reference to the dark dorsal coloration of this new species.
Distribution (Figure 5): This new species is known to be found between Bariloche and Pilcaniyeu northward to 20 km south of Cerro Alto (National Road Nº 40), Rio Negro, Argentina.
II Other terminal taxa included in the analysis
Because its distribution (too far from type locality) and some meristic characters we analyzed as terminal taxon: Phymaturus cf. punae (Road to Laguna Brava, La Rioja province). Around 40% of specimens of cf. punae examinated have their rostral scale divided like Phymaturus punae (San Guillermo, San Juan), number of scales around midbody are fewer in Phymaturus punae (x = 179.6; SD = 10.9; 168.0196) than in Phymaturus cf. punae (x = 205.8; SD = 13.7; 188.0234.0).
Phymaturus cf. antofagastensis (SC), from Cuesta de Randolfo and Sierra de Calalaste: according to Pereyra (1991) the type locality of Phymaturus antofagastensis is Los Nacimientos near Paso San Francisco. Both terminals (Paso San Francisco and Randolfo-Calalaste) have enlarged scales in the central area of chest, as described in the original description (Pereyra, 1985). Specimens from Cuesta de Randolfo and Sierra de Calalaste exhibit an undivided rostral and a homogeneous dense "spray" dorsal pattern of coloration (Figure 12C), whereas most specimens from Paso San Francisco have a divided rostral scale and an "aggregate dorsal" pattern of coloration (Figure 12D).
Phymaturus cf. palluma (CH): (Chillán, Chile): lizards from this population, as in P. cf. dorsimaculatus (Copahue, Neuquén province), have ringed tails but they lack the reticulate pattern typical of other Phymaturus. Females from this locality have immaculate throats, whereas all other P. cf. palluma have spotted or black throats. Specimens from this population also have lorilabials occasionally in contact with the subocular, which never occurs in other P. cf. palluma, and members of this population have fewer scales in contact with the nasal than in all other populations.
Phymaturus cf. palluma (EP): (El Planchón and San Pedro, Chile): females can exhibit precloacal pores (not found in other P. cf. palluma), temporal scales are protruded conical shaped not as in dorsimaculatus and P. cf. palluma (CH), anterior gular fold present (absent in P. cf. palluma of Payunia), the lorilabials never contact the subocular (as in P. cf. palluma of Chillán), there are no enlarged scales on the margins of posterior gular fold as in P. cf. palluma (ME). Specimens from El Planchón are quite similar of those from Maule (MVZ 23250607).
Phymaturus cf. palluma (ME): differs from P. cf. palluma (PA) because adult specimens of the former have partially or completely black heads and neck foldings, sexual dimorphism in dorsal pattern, but never a divided rostral (which is common in P. cf. palluma PA). This population differs also in similar way from P. cf. palluma (LB) because this last population lacks sexual dimorphism, and temporal scales in adult specimens are strongly projected (spinose).
Phymaturus cf. palluma (LB) (Laguna Blanca): is smaller than P. verdugo: 86110 mm, whereas in P. verdugo: 106120 mm SVL. From P. dorsimaculatus sp. nov. because do not exhibit the same pattern of black transversal bars over theirs neck and shoulders and ringed pattern on tails (this last character discriminate it also from P. cf. palluma CH). Does not exhibit sexual dimorphism in pattern like P. cf. palluma (ME) (with both states agregate and dense "spray" Figure 12). Is allopatrically distributed very far from the northern group of species (P. punae, P. cf. punae LR, P. antofagastensis, and P. cf. antofagastensis SC) and no individuals have a divided rostral.
Phymaturus cf. palluma (PA) (Payunia): these lizards are morphologically similar to those here called "Mendoza" but the adult specimens lack the distinct black head and neck present in other forms of "palluma". This character discriminates this form from other members of the palluma group. It shares with northern species of the group the a divided rostral in many specimens.
Phymaturus cf. patagonicus (EC) (San Antonio del Cuy, Rio Negro): Phymaturus with a gray background coloration with white spots (like in P. patagonicus from Dolavon, Chubut province), spots are smaller and usually these lizards have a dark gray to black dorsolateral band. This dark band is also present in individuals of P. tenebrosus and most females of P. zapalensis (Figure 12A), but P. patagonicus (EC) lacks black and brown morphs and has a smaller body size.
2) Phylogenetic analysis of Phymaturus comparison of four matrices
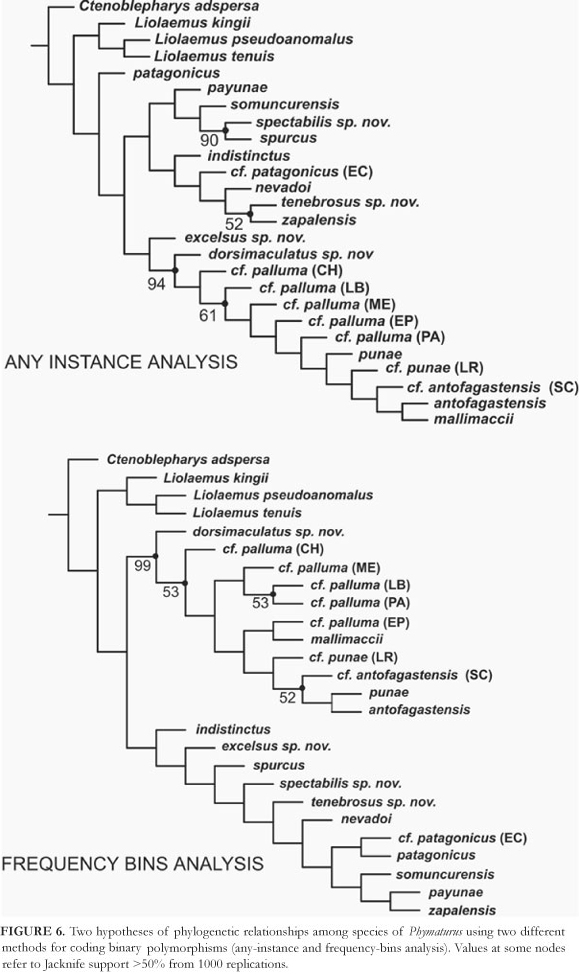
The analysis applying the ANY INSTANCE coding method for binary polymorphic characters brought one most parsimonious tree of 522.48 steps (CI = 0.57; RI = 0.66) (Figure 6). This topology recovered P. patagonicus as the most basal species of the genus, the patagonicus group is paraphyletic, P. payunae is the sister taxon of a subclade formed by P. somuncurensis, P. spectabilis, and P. spurcus, this group is related to another formed by P. indistinctus as the most basal species, P. cf. patagonicus (EC), and P. nevadoi as sister taxon to the pair formed by P. zapalensis and P. tenebrosus. Phymaturus excelsus is related to the palluma group for which P. dorsimaculatus is the most basal species. The topology of the palluma group in this tree is unbalanced, with species subsequently related as follows: P. cf. palluma (CH), P. cf. palluma (LB), P. cf. palluma (ME), P. cf. palluma (EP), P. cf. palluma (PA), P. punae, P. punae (LR), P. cf. antofagastensis (SC), P. antofagastensis, and P. mallimaccii. Jacknifing recovered four nodes with good support in the consensus tree: 52% for P. tenebrosus-P. zapalensis, 90% for P. spectabilis-P. spurcus, 94% for the palluma group, and 61% for the palluma group except P. dorsimaculatus and P. cf. palluma (CH).
Runs made applying the FREQUENCY BINS coding method for binary polymorphic characters brought one shortest tree of 978.63 steps (CI = 0.49; RI = 0.58) (Figure 6). In this analysis the patagonicus group is monophyletic with P. indistinctus as the most basal species, P. excelsus is the sister taxon to all remaining species, the subsequent node has P. spurcus as sister taxon to all the rest of the group. P. spectabilis, P. tenebrosus, and P. nevadoi are branching up the most terminal subclade formed by two groups, one formed by P. cf. patagonicus (EC) and P. patagonicus, and the other by P. somuncurensis sister taxon of the pair P. payunae-P. zapalensis. The palluma group is monophyletic with P. dorsimaculatus and P. cf. palluma (CH) as the most basal species of the group, two main subclades are formed in this hypothesis, one including P. cf. palluma (ME) sister taxon of the pair P. cf. palluma (PA)-P. cf. palluma (LB), and the other group including all northern species plus P. cf. palluma (EP). Phymaturus cf. punae (LR) is sister taxon of a clade formed by P. cf. antofagastensis (SC), P. punae, and P. antofagastensis, whereas P. mallimaccii is related to P. cf. palluma (EP). Jacknife values for this analysis include 99% for the palluma group, 53% all species of that group without P. dorsimaculatus, 53% for P. cf. palluma (PA)-P. cf. palluma (LB), and 52% for a node formed by P. punae, P. antofagastensis, and P. cf. antofagastensis (SC).
Phylogenetic analysis applying the SCALED coding method for binary polymorphic characters yielded one most parsimonious tree of 571.06 steps (CI = 0.56; RI = 0.65) (Figure 7). In this analysis the patagonicus group is monophyletic, formed by two main groups, one including P. excelsus, P. indistinctus, and P. patagonicus, and the other with P. payunae as the basal species, with P. spectabilis-P. spurcus as sister taxon of a subclade formed by P. somuncurensis, P. cf. patagonicus (EC), P. nevadoi, P. tenebrosus, and P. zapalensis. In the palluma group, P. cf. palluma (PA), and P. cf. palluma (LB) are sister taxa and basal-most subclade in the group. Phymaturus dorsimaculatus and P. cf. palluma (CH) are sister taxa, related to a central and northern clade that includes P. mallimaccii related to the pair P. antofagastensis-P. cf. antofagastensis (SC), and P. cf. palluma (EP) related to a group formed by P. cf. palluma (ME), P. punae, and P. cf. punae (LR). Jacknifing analysis of the consensus tree found support for six nodes, 64% for P. spurcus-P. spectabilis, 99% for the palluma group, 57% for all species of the palluma group except P. dorsimaculatus (not recovered in the shortest tree), 86% for P. cf. palluma (PA)-P. cf. palluma (LB), 80% for the northern clade: P. mallimaccii, P. punae (LR), P. punae, P. antofagastensis, and P. cf. antofagastensis (SC); P. cf. palluma (EP) and P. cf. palluma (ME) are not in this subclade in this consensus tree, 54% for P. punae, P. antofagastensis, and P. cf. antofagastensis (SC) (these two last taxa are related to P. mallimaccii in the shortest tree, Figure 7).
The analysis applying the MISSING coding method for binary polymorphic characters recovered two equally parsimonious trees of 489.59 steps (CI = 0.60; RI = 0.68). In Figure 7 we show one of these trees. In this analysis the patagonicus group is not monophyletic, common to these trees are the pair formed by tenebrosus and zapalensis, and the subclade including P. somuncurensis sister taxon of P. spurcus-P. spectabilis. Phymaturus dorsimaculatus and P. cf. palluma (CH) are the basal subgroup in the palluma group, P. cf. palluma (LB) is the most basal species of a big clade formed by all remaining species. Phymaturus cf. palluma (PA) and P. cf. palluma (ME) are related to a group including cf. palluma (EP) and all northern species; P. cf. punae (LR) is basal to a couple of sister taxa, one including P. punae and P. cf. antofagastensis (SC) and the other mallimaccii and antofagastensis. For this analysis Jacknifing reports eight nodes supported over 50% of frequency. The palluma group at 99%, P. cf. palluma (EP) plus all northern species: P. punae, P. punae (LR), P. mallimaccii, P. antofagastensis, and P. cf. antofagastensis at 85%, all northern species at 83%, P. cf. palluma (LB)-P. cf. palluma (PA) at 81% (not found in both trees); the subclade formed by P. punae and P. antofagastensis (SC) at 79%, P. spurcus-P. spectabilis at 68%, all northern species without P. punae (LR) at 53% and a node not found in the two shortest trees formed by P. antofagastensis, P. punae, and P. cf. antofagastensis (SC) at 74%.
Supported group common in all analysis (4) was the palluma group (94, and three 99%), P. cf. palluma (PA)-P. cf. palluma (LB) in three analysis (53, 81, 86%) (not supported in the any instance analysis). In three runs also is recovered the node P. spurcus-P. spectabilis (not in the frequency bins analysis; 52, 64, 68%). Phymaturus punae is related to antofagastensis and P. cf. antofagastensis (SC) in three jacknife consensus trees (missing, scaled and frequency) (74, 54 and 52%).The northern subclade formed by punae, punae (LR), P. mallimaccii, P. antofagastensis, and P. cf. antofagastensis (SC) have support in the missing and scaled analysis (8083%). Phymaturus dorsimaculatus is the most basal species in two of the jacknife consensus trees (frequency and scaled analysis) (53-57%).
Consensus tree and common apomorphies
In this study there are competing hypothesis that are shown in the four original topologies (Figures 6 and 7). From these four original analysis we built a majority rule consensus tree shown in Figure 8, because a majority rule tree can choose among hypothesis incongruent among the original runs observations on those contradictory relationships are given below.
Phymaturus somuncurensis is sister taxon of spurcus spectabilis (Node 5) in the any instance and missing analysis, somuncurensis is related to the pair payunae zapalensis in the frequency bins run while in the scaled analysis is basal to a group formed by other four species. Phymaturus cf. patagonicus (EC) is sister taxon of the group formed by nevadoi and the pair formed by zapalensis and tenebrosus (Node 6) in any instance and scaled runs, cf. patagonicus (EC) is sister taxon of patagonicus in the frequency bins analysis. Phymaturus dorsimaculatus is sister taxon of cf. palluma (CH) (Node 3) in scaled and missing runs, in the frequency bins run dorsimaculatus is the basal species of the palluma group not related to cf. palluma (CH). Northern species (Node 12) are present in any instance and missing runs. Phymaturus mallimaccii is related to antofagastensis in the any instance and missing runs (Node 13); mallimaccii is related to antofagastensis and cf. antofagastensis (SC) in the scaled analysis and to cf. palluma (EP) in the frequency bins run.
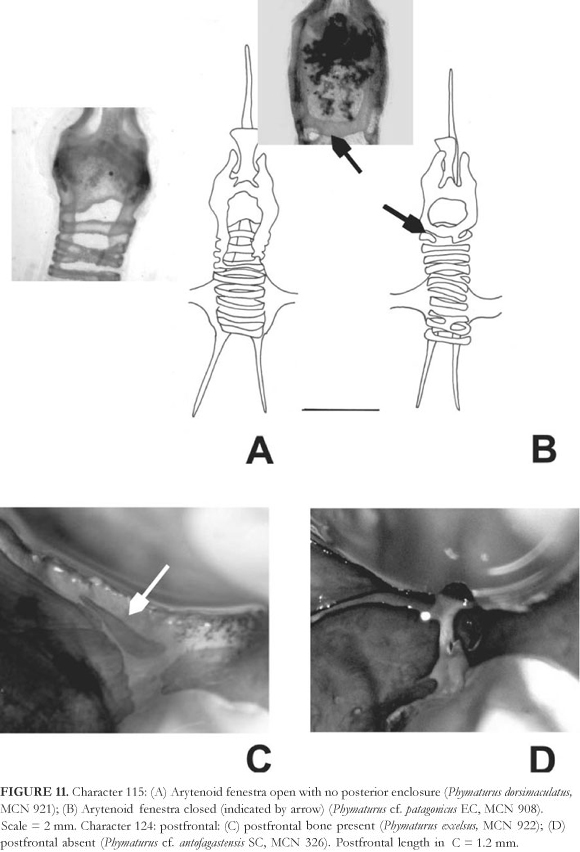
The palluma group (Node 1) is supported by the following common apomorphies (all runs) characters 5 (number of ventral scales), character 7 (number of gulars), character 11 (number of upper ciliars), character 13 (supralabial upturned), character 18 (preocular scale separation from lorilabial row) and character 26 (superciliary scales shape subcuadrangular not imbricated). The patagonicus group (Node 2) is not monophyletic in all runs and had no support as the palluma group, in those topologies where it is found monophyletic common apomorphies involves changes in characters 4 (number of dorsal scales in a head-length), 21 (males trunk length/snout vent length ratio), 28 (number of pterigoid teeth) 119 (lorilabials-subocular scale separation) and 149 (number of scleral ossicles). Evidence for the sister taxa relationship between dorsimaculatus and cf. palluma (CH) (Node 3) are changes on characters 10 (number of superciliaries) and 14 (subocular fragmentation, Figure 9). Character changes supporting Node 4 are related to characters 8 (number of scales in contact to interparietal), 13 (number of supralabial upturned), 16 (scales in contact to mental, Figure 9) and 130 (dorsal pattern of tails, Figure 10). Node 5 includes somuncurensis, spectabilis and spurcus, and changes are on characters 7 (number of gulars), 109 (male/female SVL ratio) and 151 (lacrimal foramina opening). Node 6 (within patagonicus group) is supported by changes on characters 6 (number of scales on the lateral wall of neck, between earing and shoulder) and 23 (males tibia length (SVL ratio). Node 7 (within the palluma group) is supported by the following changes, characters 9 (number of infralabials), 17 (number of scale organs on posrostrals), 18 (number of scales separating preocular from lorilabial row), 19 (maximun SVL found), 109 (male/female SVL ratio) and 128 (throat of males pattern). Characters supporting the relationship between spurcus and spectabilis (Node 8) are 14 (subocular fragmentation, Figure 9), 19 (maximun SVL found) and 110 (males trunk length/SVL ratio). Node 9 exhibit changes on characters 10 (number of superciliaries), 13 (number of supralabial upturned), 15 (scales in contact to nasal) and 18 (number of scales separating preocular from the lorilabial row). Phymaturus cf. palluma (EP) is related to the northern subclade in Node 10: characters 0 (number of dorsal head scales), 7 (number of gulars), 8 (number of scales in contact with interparietal), 17 (number of scale organs on posrostrals) and 18 (number of scales separating preocular from the lorilabial row). Phymaturus tenebrosus is sister taxon of zapalensis at Node 11, character 21 (males trunk length/SVL ratio). The northern Argentina subclade is supported by characters 6 (number of scales on the lateral wall of neck, between earing and shoulder) and 12 (number of scales between frontal and rostral). Phymaturus antofagastensis and mallimaccii are sister taxa at Node 13, characters 19 (maximun SVL found) and 110 (males trunk length/SVL ratio).
Search of additional topologies
Analyzing with the range method of TNT for continuous characters we had an unique shortest tree for three analysis (any instance, frequency bins. and scaled analysis) while running with the missing coding method for binary polymorphic characters we had two topologies. Continuous characters ranges were entered with three decimals, so length measures of trees are given with three decimals, this fact make less expectable reaching more than one topology for the same run. For this reason we wanted to know how many other topologies are close to the shortest one for each analysis, and look if exist a tree or/and trees repeated among these four different analysis. We made a search looking for suboptimal trees up to one step longer having a total of 383 topologies (any instance-analysis: 57 trees, scaled-analysis: 199, frequency bins-analysis: 29 and missing-analysis: 98). In this analysis we got three topologies repeated in two of the four runs (tree 13 of any instance-analysis = tree 369 of the missing-analysis; tree 50 of any instance-analysis = tree 84 of the scaled-analysis and tree 177 of the scaled-analysis = tree 296 of the missing-analysis) and two trees were recovered in three of the four analysis: tree 18 of any instance-analysis = tree 82 of the scaled-analysis = tree 295 of the missing-analysis; and tree 54 of the any instance-analysis = tree 176 of the scaled-analysis = tree 294 of the missing-analysis. Trees 18 and 54 repeated in three of the four analysis differs between them in the arrengement of the patagonicus group. The palluma group in these trees show the same topology obtained in the missing-analysis, for the patagonicus group recover the following topologies: ((((((((tenebrosus zapalensis) nevadoi) ((spurcus spectabilis) somuncurensis)) payunae) cf. patagonicus EC) patagonicus) indistinctus) excelsus); and the other differing only in the position of payunae, here as follows: (((tenebrosus zapalensis) nevadoi) (((spectabilis spurcus) somuncurensis) payunae)). Terminal arrengements of the patagonicus group are the same recovered in the majority rule consensus tree and in other topologies see Figures 6 to 8.
3) Observations on the ontogenetic shift of morphological characters
A sample of 15 juvenile Phymaturus cf. palluma (PA), presumably in their first range from 65.6-83.0 mm SVL allowed us to make observations on their dimorphic differentiation. Dissection of these specimens were disected to determine sex for all but two individuals. SVL X = 75.2 (SD = 4.8; range = 69.380.5) for males, and X = 75.0 (SD = 4.8; range = 67.383.1) for females. All specimens have the same dorsal pattern as adults, three of five males have their abdominal region variegated as do two of eight females. All male juveniles lack precloacal pores, but four of them (4/5) have a row of slightly modified scales suggesting the development of precloacal pores. All male juveniles have 23 enlarged scales on the base of the tail adjacent to the posterior margin of the cloacal apperture as can be seen in most adult males of the palluma group. This character is more obvious than the presence or absence of precloacal pores and be used to sex very early stages of neonate and new-born specimens without the need for dissections. Two individuals of undetermined sex were among the smallest examined: 66.1 and 65.6 mm SVL (SDSU 1955 and 1961, respectively), both lack enlarged scales on the base of the tail and no traces of precloacal pores.
Four embryos taken from two gravid dissected females of P. dorsimaculatus exhibit the following differences in comparison to adults of their species: more, smallerscales on the dorsum and flanks, counting dorsal scales in a head length (moving with the caliper a head length on the midle of the back) in these embryos are 4660 (3644 in adults), fewer scale organs on the postrostrals 23 in two embryos but not fomed in the other two (X = 2.20; SD = 0.45) than in adults (X = 3.4; SD = 1.27); temporals are flat and forming a pavement in embryos, whereas in adults these become more prominent and, in some specimens, conical; dorsal scales of the tail are smooth, whereas in adults these are rugose (as for members of the palluma group). All other characters of squamation and those referred to dorsal, ventral, and tail pattern are the same as the adults. Almost the same differences were observed between juvenile and adult P. cf. palluma (CH): more, smaller scales in the dorsum of body (3942 in juvenile versus 32 in both adult females), temporals flat in juveniles vs. conical to spiny in adults, and dorsal scales of the tail are smooth in juveniles, whereas these are conspicuously rugose in adults. There was no distinction in the number of scale organs on the postrostrals as was detected between embryos and adults of P. dorsimaculatus. Later conditions of characters (exhibited by adults) described here having change from embryo-juvenile-adult specimens like the number of dorsal body scales (in a head length), shape of the temporals, and dorsal tail rugosity are present only in species belonging to the palluma group. In species of the "patagonicus" group, those earlier stages (greater number of dorsal scales, temporals flat, tail scales smooth) for all Phymaturus species are present also in adults, and because this condition is the same in adults of Ctenoblepharys and Liolaemus, we assume that terminal additions on the ontogeny of those characters happened in the common ancestor of the palluma group.
4) Biogeography of Phymaturus
Taking into account endemism areas described for this southern region of South America (Flores & Roig-Juñent, 2001; Roig-Juñent & Flores, 2001; Roig-Juñent et al., 2002) terminals of our analysis are distributed as it follows: patagonicus, somuncurensis and cf. patagonicus (EC) in Monte Austral (MAUS); indistinctus, spectabilis, spurcus and excelsus in Patagonia Central (PCEN); tenebrosus in two areas Patagonia Central y Patagonia Occidental (POCC); cf. palluma (LB) cf. palluma (PA), nevadoi, payunae and zapalensis in Payunia (PAY); cf. palluma (ME), cf. palluma (EP) and cf. palluma (CH) in Cordillera Andina and Valle Central (CAVC); dorsimaculatus in Patagonia Occidental; punae, mallimaccii, cf. punae (LR), antofagastensis and cf. antofagastensis (SC) should be assignated to areas called Prepuna (PREP) and Puna (PUNA) for the last two species. Curiously this subclade of northern populations of Phymaturus is distributed exclusively in the transitional Puna subdistrict delimited by Martínez Carretero (1995) that occupies western region of Catamarca, La Rioja and northwestern San Juan. Spliting this group in two areas in any further biogeographic analysis (like Fitch optimization, DIVA, etc.) can carry to make extra assumptions of historical events (vicariance, extinctions or/and dispersals). Delimitation of areas among members of the northern subclade of the palluma group between Prepuna and Puna it is not enough explicit and assigning one or other area to these taxa becomes problematic. In cladistic biogeography comparing original cladograms of taxa with general area cladograms is a way to asses how our historical hypothesis are congruent with that history of the geography they occupies as it was recovered from the phylogenies of other taxa (plants or animals). Our four hypothesis for Phymaturus are incongreunt among them in many nodes of the tree, but some part of the relationships recovered are congruent to the general area cladogram published by Flores & Roig-Juñent (2001), Roig-Juñent & Flores (2001) and Roig-Juñent et al., (2002), the relationship between Monte Austral and Patagonia Central is suggested in three of the four trees: ((somuncurensis (spectabilis spurcus)) in two runs, the any instance and missing analysis, and the relationship between patagonicus and indistinctus in the scaled analysis. The relationship between Payunia and Patagonia Occidental is suggested here by tenebrosus and zapalensis in three runs, any instance, scaled and missing analysis. The relationship between Cordillera Andina Valle Central as basal of these two pairs of areas is not seen here in Phymaturus cladograms, in the four analysis species distributed in that area are close related to the norther subclade (Transitional Puna). Area relationships suggested by the frequency bins analysis is not congruent with the other three analysis of Phymaturus and with general area cladograms known. In this hypothesis (Figure 6) a repeated vicariant hypothesis is suggested within both groups of species: there exist a pair of sister taxa linking the Payunia plateau (Mendoza) with Laguna Blanca (Neuquén), cf. palluma (PA) with cf. palluma (LB) in the palluma group and other couple of species in the patagonicus group, payunae and zapalensis.
In Figure 8 a majority rule consensus tree of the four original analysis show species of Prepuna and Puna areas of the palluma group forming a monophyletic group with cf. palluma (EP) inhabiting Valle Central and Cordillera Andina as the sister taxon. Phymaturus dorsimaculatus found in Patagonia Occidental is sister taxon of cf. palluma (CH) a Chilean species distributed not far from this locality. Phymaturus cf. palluma (LB) distributed at southern latitude as dorsimaculatus and cf. palluma (CH) is the subsequent basal species. Western central Argentina species as cf. palluma (ME) and cf. palluma (PA) are more related to the cf. palluma (EP) and the northern clade than any other. In the patagonicus group there exist a repeated pattern where Monte Austral was related to the patagonian areas, at Node 5 somuncurensis basal species of this clade, and cf. patagonicus (EC) basal to the other clade.
DISCUSION
Taxonomic remarks
The taxonomic use of palluma or flagellifer for designating the oldest known species of the genus should be resolved soon by the International Commision of Zoological Nomenclature (ICZN). There are two competing positions: Cei & Lescure (1985) and Lescure & Cei (1991) proposed the use of flagellifer instead of palluma Molina, 1782, owing to confusion involving the type specimen, which was an individual of the Chilean teiid Callopistes maculatus. Recently, Etheridge & Savage (2003) proposed conservating the usage of the name palluma by designation of a neotype for Lacerta palluma Molina, 1782, because its wide use in the literature for the last 150 years. Both requests are reasonable and will be considered by the ICZN in 2005.
The name Phymaturus adrianae, proposed by Pereyra (1992b) for a new species in an meeting abstract is a nomen nudum (ICZN, Art. 9, 1999). However, Cei & Videla (2003) used the name and provided a photograph and some characteristics to diagnose the species, and thus may be considered the authors of the name Phymaturus adrianae. But because there was no type material indicated in this publication, we do not consider this name valid. Considering that at this time we do not know certainly the identity of the true palluma (if it is assignable to populations of Mendoza in Argentina or to populations of Chile, as noted by Cei & Videla, 2003) exist the risk that adrianae (populations of Argentine side of the Andes) could be a synonym of palluma. The aim of this study was extracting phylogenetic information from morphological characters and the description of new taxa, the resolution of those taxonomic problems requires others kind of information.
In this study we reexamined materials from Baños del Campanario (Talca), Puesto Militar San Pedro, Cuesta Vergara (Curicó), El Planchón (Curicó) at MNHN and Paso de los Cóndores, Laguna del Maule (Talca) (deposited at MVZ). We considered those populations to be conspecific because we coud not find obvious morphological differences. Similar conclusions can be addressed following the cytogenetic studies done on these populations. Lamborot & Navarro Suárez (1984) studied materials from Cordillera de Curicó close to El Planchón & Pereyra (1992a) examined Phymaturus flagellifer from the same region: Termas del Flaco (Curicó), Mina la Disputada (La Colina), Laguna del Maule (Talca). Both cytogenetic studies provided the same results.
Recently Scolaro & Cei (2003) described a new species of Phymaturus based on a single specimen collected in the area of Esquel (Chubut, Argentina). Characters provided in this description make it difficult to assign this new taxon to either species group, but some comments are warranted. According to their description, the specimen has imbricate superciliaries (pg. 109) and a spiny tail, but not as spiny as that seen among members of the palluma group (pg. 111). These characters suggest this new species is allied outside of the palluma group, but the authors also describe the new taxon as possessing four suboculars separated from the supralabials by two rows of lorilabials a character that is shared by all members of the palluma group. However, fragmentation of the subocular was also described for P. spurcus (Lobo & Quinteros, in press). The dorsal pattern of this lizard resembles specimens of P. tenebrosus and some P. zapalensis, as well as other species of the "patagonicus" group: P. excelsus, P. spectabilis, P. tenebrosus, and P. spurcus, which exhibit orange-red coloration on their abdominal region. Given this evidence, it seems unlikely that this new taxon is nested within the palluma group. A more careful examination of the material and the collection of additional specimens should help to resolve this question.
The new species described in this investigation have distinctive color patterns, and all (except P. excelsus) are allopatric.Phymaturus tenebrosus and P. spectabilis exhibit an interesting polymorphism in pattern, but they do not resemble other species of the genus. Some specimens of P. tenebrosus have a brown background coloration with irregular black spots, which becomes increasingly melanistic in intermediate specimens reaching the completely black pattern. A few specimens of P. spectabilis have a brown background coloration (without typical white occellation) with irregularly located small markings on the dorsolateral region of the trunk The only species living syntopically are P. excelsus and P. spurcus, yet no intermediate individuals were found at Ojo de Agua. Rocky formations in western Argentina and Patagonia are disjunct, forming small patches (of a few kilometers of area)that isolates these populations of lizards, which are very restricted in their microhabitat preferences (restricted to rocky outcrops). Other terminals included in this study (as "cf.") are the subjects of current studies associated with determining their taxonomic status.
Phylogenetic conclusions
Using different coding methods for analyzing polimorphisms can affect results and can yield to different topologies. Systematists analyzing morphological information should not avoid using polymorphic or/and continuous characters and should pay more attention in methods they apply (not just one as we can see in most of published analysis). Studying congruence between our results and others constructed from different (independent) evidence (e.g., DNA) could provide tests of the accuracy and performance of these different approaches to understanding morphological variation. Another way of evaluating these competing hypothesis would be applying reconcilied trees (Page, 1994; Page & Charleston, 1998) or other alternative methods for studying the biogeographic history of a group of organisms, those hypothesis that implies fewer hypothesis of events (dispersals, extinction, etc.) would be the preferred ones.
The palluma and patagonicus groups The palluma group is strongly supported in this analysis (always over 90% of jacknife values), so there is no doubt about its monophyly. Characters supporting the palluma group are not seen in the other two genera of Liolaemidae. There are three additional characters that could be considered apomorphies for this group (that should be revisited in future studies): all members of the group exhibit a longitudinal central band in the dorsum of sligthly enlarged scales that are larger than those along the flanks. This character is not exhibited by species of the patagonicus group. Scales of the tail are spinier than those of the patagonicus group and have surface rugosities, which are absent in members of the patagonicus group. Scales of the tail of Phymaturus patagonicus are not more spinose than many species of Liolaemus, a closer observation of scale shape and arrangements reveals that the difference between the genera is not the size of spines but their shape (subquadrangular/rectangular in Phymaturus, round to lanceolate in Liolaemus) and their disposition, forming more conspicuous rings around the tail perimeter in Phymaturus. Characters states given by Etheridge (1995) for the patagonicus group are shared with Liolaemus or exhibit variation within that genus, so it is not surprising to find that this group lacks support in our analysis or was even found to be paraphyletic in some analyses (see Figures 4 and 5).
Relationships within the palluma group In all analysis performed northern species of the palluma group (P. punae, P. cf. punae LR, P. antofagastensis, P. cf. antofagastensis SC, and P. mallimaccii) formed a monophyletic group, and in all topologies P. cf. palluma (EP) is nested within the group or as the sister taxon of the group. Phymaturus dorsimaculatus is the basal species of the group. Cei & Videla (2003) described a pattern of transverse black bars over the dorsum of specimens of P. verdugo, we were not able to examine those specimens, but this character suggests a relationship with P. dorsimaculatus. In some analysis Phymaturus cf. palluma (CH) falls as sister taxon to P. dorsimaculatus or occupied a basal position in the palluma group. Both species have ringed tails and their distribution are proximate. However, larger samples of P. cf. palluma (CH) are needed for study. The position of P. cf. palluma (ME), P. cf. palluma (PA), and P. cf. palluma (LB) are uncertain, in some analysis they form a monophyletic group, yet in others they are not closely related and represent subsequent diverging branches in the evolution of the group previous to the origin of the northern subclade.
Relationships within the "patagonicus" group Within this "group" relationships are problematic.Few of the internal nodes were well supported and the competing hypothesis indicate that much additional work is needed. Relationships between P. spurcus and P. spectabilis, like those found between P. tenebrosus and P. zapalensis, were paired in several analyses, but not in every case (see frequency bins analysis, Figure 6). Even without specific analysis of levels of polymorphism and degree of overlap of continuous characters across the genus, preliminary observations suggest that the patagonicus group is more evident and that fact can affect more the morphological analysis than in the palluma group. Perhaps in the patagonicus complex radiation of independent lineages was fast and more recent, not giving sufficient time for the accumulation of morphological differentiation. Overall, we found more divergent patterns of morphological variation within the patagonicus group than in the palluma group (see Figures 3 and 4), yet we found less differentiation in other types of characters (squamation, skeleton, etc.).
Sampling limitations This first morphological approach to recovering the phylogeny of Phymaturus is based on a heterogeneous assemblage of evidence. First, it is obvious that the alpha-taxonomy of the genus is less studied and known than in many groups of Liolaemus, so it seems likely that many more terminal taxa should be included in future analyses(including the recently described species P. verdugo and P. calcogaster). Second, we had incomplete information from allozymes (only for 27% of terminals) and skeletons (only 59% of terminals). In some cases we had only a small number of specimens (i.e., P. cf. palluma LB, P. cf. palluma CH; P. nevadoi, only known from three specimens), which can affect results of the phylogenetic analysis (Hillis, 1998). One of our goals for future studies will be increase these samples both in characters and taxa.
Comparisons to a previous revision of the genus The main goals of this investigation were proposing hypothesis about the history and phylogenetic relationships within the genus Phymaturus based on derived shared characters. The most extensive recent study on Phymaturus was conducted by Pereyra (1992a), he studied 35 meristic, 52 morphometric, 19 enzymatic, and 4 chrommosomal characters for six especies including in his clustering analysis five of them. Because Pereyra's (1992a) study was conducted using phenetic criteria it is difficult to compare his results with ours, yet some observations are warranted. Even when a phenetic analysis only show degree of similarity without discriminating similarities originated by common ancestry from homoplasy or plesiomorphy, Pereyra (1992a) made phylogenetic conclusions including a proposed common ancestry for P. antofagastensis, P. mallimaccii, and P. payunae (pg. 99), saying that conclusion is consistent with phenograms obtained. He adds to his conclusions that the rest of "flagellifer group" species as derived from close ancestor to the P. antofagastensis-P. mallimaccii node which is contradictory with the previous sentence. His separated phenetic analysis of meristic, morphometric, chromosomal and biochemical data show incongruent results among them. The meristic analysis of Pereyra (1992a) found payunae as the most distant phenetic species ((((P. antofagastensis-P. punae) (P. cf. palluma ME P. cf. palluma CH)) P. mallimaccii) P. payunae) (fig. 15, pg. 36) while its morphometric data- based phenogram shows ((((antofagastensis cf. palluma ME) P. mallimaccii) P. punae) P. payunae) cf. palluma CH) with P. palluma from Chile as the most distant phenetic species (fig. 24, pg. 47). Pereyra's chrommosomal analysis found the node P. payunae-P. cf. palluma CH (fig. 38, pg. 61) linked to ((P. antofagastensis-P. mallimaccii ) P. cf. palluma ME) and his analysis of enzymes found P. payunae-P. cf. palluma ME (fig. 39, pg. 64) linked to ((P. antofagastensis-P. mallimaccii ) P. cf. palluma CH). Thus, his own results as was shown above do not allow conclusions as those proposed. Beyond systematic or/and phylogenetic conclusions that circumstantially we can agree or disagree, Pereyra's (1992a) study brings valuable information that we already re-analyzed in the context of our cladistic analysis (see methods section).
Biogeographic Implications
Morrone (1994, 1996) analyzed relationships among provinces of the Andean subregion (corresponding to southern South America below 30° South latitude) based mainly on arthropod fauna. He found subantarctic and central Chilean provinces as sister taxa and more closely related to Puna province. In our analysis, P. cf. palluma (EP) inhabiting Cordillera andina and Valle Central (circa Santiago latitude) is related to species of Prepuna and Puna, whereas P. cf. palluma (CH) (southern populations) and P. cf. palluma (ME) distributed in the Argentine province of Mendoza (eastern Andean mountains) are related to other "palluma" of Payún and central Neuquén. Recent analysis of the chiliensis group of Liolaemus (Lobo, in press) found species groups living mainly at Valle Central (L. gravenhorsti and L. robertmertensi) more related to the alticolor group (Puna) than to any other group.
An obvious conclusion of this study is that more taxonomic and phylogenetic studies of Phymaturus are needed. We are confronted with a group that is substantially more species rich than originally thought and because their distribution and peculiar natural and life history (saxicolous, herbivorous, and viviparous) offers a very interesting and tempting challenge to herpetologists, both systematic and ecological.
ACKNOWLEDGMENTS
We thank R. Espinoza for making valuable comments on an earlier draft of the MS and his help with its translation. We would like to thank the following individuals (and museums) for allowing us to study specimens under their care: E. Pereyra (Instituto de Biologia Animal, Universidad Nacional de Cuyo, Mendoza), F. Videla (IADIZA, Mendoza), E. Lavilla and S. Kretzschmar (Instituto de Herpetologia, Fundacion Miguel Lillo, Tucumán), H. Núñez (Museo Nacional de Historia Natural, Santiago), R. Etheridge and T. Reeder (San Diego State University), J. Hanken and J. Rosado (Museum of Comparative Zoology, Harvard), J. Wiens (Carnegie Museum of Natural History, Pittsburgh), J. McGuire (Museum of Vertebrate Zoology, Berkeley). We thank F. Cruz, N. Ibargüengoytía, J.C. Acosta, J. Wiens, R. Espinoza, L. Avila, M. Morando, I. Martínez Oliver, C. Abdala, and J.M. Díaz Gómez for helping us in the field, in the lab, or discussing ideas related to this study. We thank S. Kretzschmar for helping us with the translation of our abstract to Portuguese. We acknowledge the Provincial Departments of Fauna of Argentina for providing authorization for collecting and field work. The senior autor thanks C. Hitchcock, Andrea and José Rosado, Carlos and Monica Calvo, and Richard Etheridge for their hospitality and kindness during a study trip in the United States. This study was supported by a grant (FL) from Agencia Nacional de Promocion Cientifica y Tecnologica (PICT 984867) and from a National Geographic Society grant to R. Espinoza and J. Wiens.
Recebido em: 10.01.2005
Aceito em: 29.08.2005
Skeletonized specimens are indicated by CS (claired and stained skeletons) and with DS (dry skeletons).
Phymaturus antofagastensis: SDSU 1991. Argentina: Prov. Catamarca: Dpto Antofagasta: Agua de los Pocitos. E. Teran & O. Pagaburo cols. 28.XI.81. MCN 309310. Camino a Paso San Francisco, Abdala, C.; R. Espinoza, F. Lobo & M.I. Martínez Oliver. MCN 14291436. A 130 km de Fiambalá sobre ruta a Paso San Francisco. Dpto. Antofagasta, Prov. de Catamarca, Argentina. J.C. Acosta col. Phymaturus cf. antofagastensis (SC): MCN 32327, 306308. Argentina, Cuesta de Calalaste, Dpto. Antofagasta de la Sierra, Prov. Catamarca. Abdala, C.; R. Espinoza, F. Lobo & M.I. Martínez Oliver. 20/01/01. MCN 313322. Argentina, Cuesta de Randolfo, Prov. Catamarca. Abdala, C.; R. Espinoza, F. Lobo & M.I. Martínez Oliver. 18/01/01.
Phymaturus dorsimaculatus (aditional materials not considered as paratypes). MVZ 232503. Depto. Ñorquin, Barda W Termas de Copahue; elevation 2050 m. Prov. Neuquén, Argentina, M.I. Christie. 29/12/94. MCN 156667 and MCN 148788 (CS) Copahue, Dpto. Ñorquin, Neuquén, Argentina. D. Pérez col. 01/99. Phymaturus indistinctus: IBA 6661 (holotype), IBA2, IBA3. 2 km O de Las Pulgas (lago Munsters), prov. del Chubut, Argentina. 700800 m. J.M. Cei & L. Cei cols. 23/1/70. MCN 127477. Las Pulgas (cerro frente a Gruta de la Virgen). Dpto. Sarmiento, Prov. de Chubut, Argentina. Abdala, C.; F. Lobo; I. Martinez; S. Quinteros cols. MCN 810 (CS) Las Pulgas, 50 km al SO de Lago Munster. L. Avila col. Ruta Prov. 20, Sierra de San Bernardo, 19 km W Los Manantiales, 669 m. 45°27'41"S; 69°42'52"W. Dpto. Rio Senguer, Prov. de Chubut, Argentina. L. Avila & M. Morando cols. MCN 1482 (CS) Ruta Prov. 20, 4 km N de intersección ruta prov. 22, 502 m. 45°25'54"S; 69°50'25"W. Dpto. Rio Senguer, Prov. de Chubut, Argentina. L. Avila & M. Morando cols. Phymaturus mallimaccii: REECSUN 183, 489491.Cueva de Perez, Sierra de Famatina, Prov. de La Rioja. Argentina. R. Espinoza & F. Cruz cols. MCN 920 and MCN 148384 (CS). Camino a la Mejicana, 3430 m. 28°54'43"S; 67°42'47"W. Dpto. Famatina. Prov. de La Rioja. Morando, M.; L. Avila y L. Belver cols. 7/12/99. Phymaturus nevadoi: IBA 999 (3 specimens) (type series). Agua de la India Muerta, 1750 m, Macizo Nevado, SE Mendoza. XII/73. J.M. Cei, J. Williams, Stassi, Castro cols. Phymaturus cf. palluma (ME): SDSU 19691970. Argentina: Prov. Mendoza: Dpto Las Heras: 20 km NE Uspallata, 2500 m. R. Etheridge. 26.I.83. SDSU 3387. Argentina: Prov. Mendoza: Dpto La Heras: 27 km NE Uspallata. 32°28'52.2"S-69°09'59.2"W. 2768 m. R. Etheridge, R. Espinoza, S. Torres, E. Pereyra. 10.II.95. SDSU 3388. Argentina: Prov. Mendoza: Dpto La Heras: 27 km NE Uspallata. 32°28'52.2"S-69°09'59.2"W. 2768 m. R. Etheridge, R. Espinoza, S. Torres. MVZ 126991. Dpto Malargüe, Valle Hermoso, Prov. De Mendoza, Argentina. R. Sage col. 5/1/69. 35°20'S; 70°15'W. MVZ 12699294. Lago de la Niña Encantada. 6 km E de los Molles, elevation 2000 m. Prov. De Mendoza, Argentina. R. Sage. 9/1/69. 33°18´S; 69°83´W. MVZ 126995. Dpto Malargüe, en el extremo norte del Valle Hermoso. Prov. De Mendoza, Argentina. R. Sage. 12/1/69. 35°11'S; 70°10'W. MVZ 126996126999. Depto. Tupungato, Quebrada de Chupasangral, 4 km NW Cerro Chupasangral; elevation 2800 m. Prov. Mendoza, Argentina R. Sage. 28/1/69.33°21'S; 69°51'W. MVZ 127023. Depto. Las Heras, 2 km E Los Hornillos, Prov. Mendoza, Argentina. R. Sage col.13/2/70.32°51'S;68°99'W. MVZ 12702527. Depto. Malargüe, 2 km E Agua Botada Prov. Mendoza, Argentina R. Sage col.24/3/70. 35°62' S; 69°95' W. MVZ 145146. Depto. Las Heras, Pampa de Canota, 20 km E, 8 km S Estancia Uspallata; elevation 3000 m. Prov. Mendoza, Argentina. R. Sage col.12/11/68. 32°65'S; 69°27'W. MVZ 18077174. Depto. San Carlos, Quebrada Cruz de Piedra. Prov. Mendoza, Argentina. R. Sage. 28/12/75. 34°26'S; 68°90'W. MVZ 92902, 04, 08. (DS) Dpto. Las Heras, Mendoza, Argentina. 16/XII/67. R. Sage col. REESDSU 230607, 231213, 2315 (DS). 20 km NE Uspallata, 2500 m. R. Etheridge col. 26/1/83. IADIZACH. S/N (2 specimens) Paramillos, Prov. de Mendoza, Argentina. IBA 760 (4 specimens). Paramillos, Mendoza. 2000 m. Argentina. 1/71. L. G. Castro col. Phymaturus cf. palluma (Maule, Chile) MVZ 23250607. On the road to Laguna del Maule (Los Condores Pass), Talca Prov.; elevation 1800 m. Region VII (= Region del Maule), Chile. R. Sage col. 18/4/87. Phymaturus cf. palluma (PA): SDSU 194851, 56, 62 6465. Argentina: Prov. Mendoza: Dpto Malargüe: 3 km NW of base of Volcán Payún. R. Etheridge. 4.II.83. SDSU 1972, 197475. Argentina: Prov. Mendoza: Dpto Malargüe: 10 km south of base of Volcán Payún. R. Etheridge.4.II.83. REESDSU 232327 (DS). 4 km W Base Volcán Payún, 200 m, Dpto. Malargüe, Prov. de Mendoza, Argentina. R. Etheridge col. 4/II/83. IADIZACH 00091. Base del Volcán Payún. 18002000 m., Prov. de Mendoza, Argentina, J.M. Cei 7 F. Videla cols. 31/1/82.IBA 733 (5 specímens). Base Campamento. Lado SW del Payún. Mendoza. Argentina. 06/01/71. L.P. Castro col. Phymaturus cf. palluma (CH). MVZ 19943538 & 230992. Hotel Termas de Chillán. Region VIII (= Region del Bío Bío), Chile. J. H. Carothers col. 1/12/1985. MCZ 165456. Cordillera de Chillán. Chile. G. Moreno col. 25/02/78. MCZ 169935. Chile. Philippi col. Phymaturus cf. palluma (LB): IBA 793 (4 specimens). Laguna Blanca. Neuquén, Argentina. J.M. Cei, L. Cei & R. Ferreira cols. 6/01/72. MVZ 23250405. Puesto Control, 3.5 km N Co. de 1 Laguna PN Laguna Blanca. 23°80'S; 56°83'W. Dpto. Zapala, prov. de Neuquen, Argentina. 1800 m. 18/03/94. M.I. Christie col. SDSU 1971. Argentina: Prov. Neuquén: Dpto Zapala: south shore of Laguna Blanca. R.E. Etheridge col. 22/02/83.
Phymaturus cf. palluma (EP): MNHN 2352, 246061. Baños del Campanario (1500 m), Talca, San Clemente. 23/2/1992. J.C. Torres-Mura. MNHN 350509. Curicó Puesto Militar San Pedro, Pichuante, Cuesta Vergara. Chile. 35°10'S; 70°36'W. H. Núñez & A. Labra cols. 27/02/84. MNHN 163233, 1638, 1643. El Planchón (Int. Curicó). 26/II/1984. M.A. Labra and H. Núñez cols. Phymaturus patagonicus: MLP 778, 777 (sintypes). Territorio del Chubut (Patagonia). SDSU 1980. Argentina: Prov. Chubut: Dpto Gaiman: 40 km WSW Dolavon. Retheridge col. 25.II.83. IADIZACH 00080. 40 km W Dolavon, 350 m. Prov. del Chubut, Argentina. J.M. Cei & J. Williams cols. 3/3/83. IBA 789 (7 specimens). Argentina: Prov. Chubut: Dpto Gaiman: 40 km W Dolavon. Cei J.M.; L. Cei & L. Ferreyra cols. 16/01/72. MCN 12841286. A 40 km al Oeste Dolavon. Dpto Gaiman, Prov. de Chubut, Argentina. Abdala, C.; F. Lobo; I. Martinez; S. Quinteros cols. 10/2003. IBA 783 (5 specimens). 20 km. W Sombrero. Prov. del Chubut, Argentina. 15/1/72. J.M. Cei, L. Cei & R. Ferreira cols. FML 1007785. 1 km W Interseccion Ruta Prov. 53 y 90. 2,2 km SW Meseta El Sombrero. Pto. Paso de Los Indios, Prov. del Chubut. C. Abdala, R. Espinoza & J. Wiens cols. 16/2/01. MCN 125058, 1261. Cerro frente a El Sombrero. Dpto. Paso de Indios. Prov. de Chubut. Argentina. Abdala, C.; F. Lobo; I. Martinez; S. Quinteros cols. 10/03. Phymaturus cf. patagonicus (EC): MCN 910918. Argentina, Prov. de Rio Negro, Dpto. 25 de Mayo, Ruta Prov. 8, 17 km S de San Antonio del Cuy. L. Avila; M. Morando & D. Pérez cols. 11/03/99. MCN 908909 (CS). Ruta Provincial 8. A 17 kms S de San Antonio del Cuy. Prov. Rio Negro, Argentina. 40°17'13"S; 68°27'32"W. L. Avila col. Phymaturus payunae: IBA 769 2,48, 10, 12, 17, 20, 24, 26. (specimens of type series). Payún Plateau, 5 km from the Volcán Payún, 2000 m, Mendoza, Argentina. Cei, J. M., L.P. Castro and T. Ferreyra cols. XII/1971. IADIZACH 000878, 000879. 20 km SE Volcan Payún. 1800 m. Cei & Videla cols. 31/01/82. SDSU 19811984. Argentina: Prov. Mendoza: Dpto Malargüe: 10 km SW base of Volcán Payún. R.E. Etheridge col. 4.II.83. MCZ 15207981. Basaltic rocks of the Payún plateau, Mendoza, Argentina. L.P. Castro col. 01/72. REESDSU 233032, 2339, 2360 (DS). 10 km S base Volcan Payun, Dpto. Malargüe, Prov. de Mendoza. Phymaturus punae: MCZ 19217 (Holotype). 7 km SE refuge de la Reserva Provincial, cerca del Rio San Guillermo, 3500 m. Prov. de San Juan, Argentina, R. Etheridge, J.M. Cei & F. Videla cols. 9/02/83.MCZ 163982,84,8688. (paratypes). Same data of holotype. SDSU 197879. Argentina: Prov. San Juan: Dpto Iglesia: Llano de los Hoyos, Reserva Prov. San Guillermo. R.E. Etheridge col. 9.II.83. REESDSU 235657 (DS). Caserones, 4 km SW refuge, Res. Prov. San Guillermo. 3500 m. Dpto. Iglesia, prov. de San Juan, Argentina. 10/II/83. R. Etheridge col., 238384 (DS). Llano de Los Hoyos, 10 km SE refuge, res. Prov. San Guillermo, 3400 m. 9/II83. R. Etheridge col. Phymaturus cf punae (LR): REECSUN 270271, 504508. Agua Quemada, Puesto Leoncito. Reserva Laguna Brava, Prov. de La Rioja. R. Espinoza & F. Cruz cols. FML 29252, 4, 811,13. Puerta Quebrada del Leoncito, camino a Laguna Brava, 57 km de Alto Jagüel, Dpto. Sarmiento, Prov. de La Rioja.1415/11/91. FML 2926.13. Agua Quemada, camino a Laguna Brava, Alto Jagüel, Dpto. Sarmiento, Prov. de La Rioja. O. Pagaburo & Bracamonte cols. 01/93.
Phymaturus somuncurensis: IBA 470 (2 specimens). (Types). Laguna Raimunda, Meseta de Somuncurá, Prov. de Rio Negro. Argentina. Cei. & Tuzi cols. 10/4/68. IBA 507 (4 specimens). Circa Laguna Raimunda, Meseta de Somuncurá, Prov. de Rio Negro. Argentina. Cei, Castro & Tuzi cols. 17/11/68. IADIZA 212. Cerro Corona, Meseta de Somuncurá. Prov. de Rio Negro. Argentina. J. Scolaro col. 16/12/85. SDSU 17801783. Argentina: Prov. Río Negro: Dpto 9 de Julio: 2 km N Laguna Raimundo, Meseta Somuncurá, R.E. Etheridge col. 19.II.92. REESDSU 24332435, 2439 (DS). N Laguna Raimundo, Meseta de Somuncura, Dpto. Valcheta, Prov. de Rio Negro, Argentina. R. Etheridge col. 19/II/83. IADIZACH 00212. Co. Corona. Meseta de Somuncura. Prov. de Rio Negro, Argentina. J.A. Scolaro col. FML 8435. 43 km al N de Moligüe. Dpto. 25 de Mayo, Rio Negro. 41°35'S; 69°22'W. F. Cruz col. 15/03/99. 16/XII/85. FML 1038. Meseta de Somuncurá, Laguna Raimundo (1400 m). Prov. de Rio Negro. Argentina. J.M. Cei col. 19/02/83. MCZ 156909, 17044344. Laguna Raimunda, Meseta de Somuncurá, Prov. de Rio Negro. Argentina. Cei. & Tuzi cols. 10/4/68. Phymaturus spurcus: MCZ 14791 (holotype). Huanuluan, Prov. de Rio Negro, Argentina. J.L. Peters col. 1920. MCZ 1491415 (paratypes) same data of holotype. MCN 123840, 124449. Cerro frente Estancia Huanuluan. Ruta 23 a 22 km al Oeste de Jacobacci Abdala, C.; F. Lobo; I. Martinez; S. Quinteros cols. 10/2003. MVZ 18890407. Depto. Ñorquinco, along Rimrock, 4 km S and 1 km E Alto del Escorial; elevation 1100 m. Prov. Río Negro, Argentina. R. Sage col. 25/2/82. MCN 1385, 1387. Ojo de Agua. Ruta 6. Dpto. Ñorquinco, Prov. de Rio Negro, Argentina. Abdala, C.; F. Lobo; I. Martinez Oliver; S. Quinteros cols. MCN 1590. Ruta prov. 6, 1 km NW de Ojo de Agua, 1141 m. Dpto. Ñorquinco, Prov. de Rio Negro, Argentina. L. Avila & M. Morando cols. 41°32'30"S; 69°51'33"W. Phymaturus tenebrosus (aditional materials not considered paratypes): MCN 15911595, 15971599. Entre Bariloche y Pilcaniyeu, Prov. de Rio Negro, Argentina. N. Ibarguengoytia col. MCN 18992 (CS) Entre Bariloche y Pilcaniyeu. Prov. de Rio Negro, Argentina. N. Ibargüengoytia col. Phymaturus zapalensis: IBA 792 (type series, 4 specimens). Laguna Tern (L. Blanca). Prov. de Neuquen, Argentina. 6/1/72. J.M. Cei, L. Cei & R. Ferreira cols. IBA 8661 & 9983 (2 specimens. 55 km S Piedra del Aguila, Neuquén, Argentina. 21/1/73. SDSU 19851988. Argentina: Prov. Neuquén: Dpto Zapala: S. shore Laguna Blanca. R.E. Etheridge. 22.II.83. MCN 160002 and MCN 148586 (CS). 1 km al S del Salitral, Ruta nac. 40. Dpto. Catán Lil, Neuquén. 39°40.600' S; 70°36.925' W; 994 m. C. Abdala, R.E. Espinoza, & J. J. Wiens cols. 10/02/01. SDSU 198990. Argentina: Prov. Neuquén: Dpto Zapala: S shore Laguna Blanca, 1275 m. W. E. Duellman. 18.XII.74. MVZ 23250812. R. Prov. 46, 1580 m. 9.5 km S, 5 km Co Chachil, Dpto. Catan Lil, Prov. de Neuquen, Argentina. M.I.Christie col. 22/01/96. MVZ 232514. Puesto de Control, 3.5 km N. de Co. de 1 laguna. PN Laguna Blanca (23°80', 56°83'). Dpto. Zapala, Prov. de Neuquen. Argentina. 1300 m. M.I. Christie col. 18/03/94. MVZ 23251516. Ruta provincial 46, Dpto. Zapala, Prov. de Neuquen, Argentina. M.I. Christie col. 16/03/94. MVZ 232513. 1/2 km W Primeros Pinos, 1600 m. Dpto. Pirunches. Prov. de Neuquen. Argentina. M.I. Christie col. 20/01/96. REESDSU 24512453 (DS). Margen sur de Laguna Blanca, Dpto. Zapala, Prov. de Neuquen. R. Etheridge col. 22/II/83. MVZ 18890810. Depto. Huiliches, rocks along Rio Malleo, 8 km N and 4 km E Junin de los Andes; elevation 800 m. Prov. Neuquén, Argentina R. Sage col. 23/11/82. 39857104. Ctenoblepharys adspersa: SDSU 3781. FML 0368, 0464.Ciudad de Dios Perú. Weyrauch col. 1/56. REESDSU 2513 (DS). Playa Ventanilla cerca de Lima. 11/XII/50. Peru. LACM 49147 (DS). Dpto. Ica. Museo Peracas. 30.2 km SW Pisco, 7.2 km SW Peracas. Liolaemus kingii: SDSU 167071, 3378. MCZ 150291. Golfo de San José, Pen. Valdéz, Chubut, Argentina. MCZ 11837, 3940. Patagonia. Hatcher col. MCZ 1894849. Ultra Cautín, Prov. Cautín Chile. C. Reed col. MCN 154550. Rio Seco, Ruta Nac. 3 entre San Julián y Tres Cerros, S 48°31.817'; O 67°44.081'. C. Abdala, P. Cacivio, L. Federico & Lobo cols. MCN 155152. Tres Cerros, S48°07.160';O67°38.384'. C. Abdala, P. Cacivio, L. Federico & Lobo cols. MCN 1324. Las Pulgas (Cerro frente a Gruta de la virgen) Dpto. Sarmiento, Prov. Chubut, Argentina. Abdala, C.; F. Lobo; I. Martinez Oliver; S. Quinteros col. MCN 565568 (CS). Sin datos. Liolaemus tenuis. MACN 36558. Costa Sur, lago Ñorquinco. 1050 m. PN Lanín, Neuquén. Argentina. I. Belke col. MACN 36559.12 km al El Lago Pilhué, 1075 m. PN Lanín, Neuquén. Argentina. MACN 36560. Extremo SW Lago Ruca Choroi, 1250 m, PN Lanín, Neuquen. Argentina. MCN 531 (CS). 1190 m. Ruta Provincial N°11, 2 km O. de Arroyo Remecó, 39°02'59''S; 71°21'32''O. Dpto. Aluminé, Prov. de Neuquén, Argentina. MCN 532533 y 518 (CS). Ruta Prov. 23, 8 km. N. Pilolil, orillas Río Aluminé. 39°32'29''S; 70°57'21''W, Dpto. Catán Lil, Prov. de Neuquén, Argentina. Liolaemus pseudoanomalus. MCN 526527 (CS). 6 km. E de Anillaco (28°47'S; 66°52'O), Dpto. Castro Barros, Prov. de La Rioja, Argentina. MCN 529 (CS). Mismos datos. MCN 528 (CS). 4 km. E de Anillaco (28°47'S; 66°52'O), Dpto. Castro Barros, Prov. de La Rioja, Argentina. MCN 530 (CS). 6 km. E de Anillaco (28°47'S; 66°52'O), Dpto. Castro Barros, Prov. de La Rioja, Argentina. MCN 1603. Sin datos. MCN 16361637. 4 km. E de Anillaco (28°47'S; 66°52'O), Dpto. Castro Barros, Prov. de La Rioja, Argentina.
List of Characters
The following characters are listed according to their position in the analyzed data matrix: characters 0–25 are apomorphies described by Etheridge (1995) for Liolaemus (07), Ctenoblepharys (819), and Phymaturus (2025). Characters 26 and 27) diagnose the palluma and patagonicus groups of Phymaturus (Etheridge, 1995). One character cited by Etheridge (1995) as an apomorphy of Phymaturus was eliminated from the list (lateral nuchal sacs filled with fat) because this character is also present in some species of Liolaemus (i.e., basal species in the chiliensis group, Lobo, 2001). Another character (number of sternal ribs) was included in the skeletal characters because of variation (34 ribs). We selected only two chromosomal characters because the number of meta- and submetacentric chromosomes and the number of telo- and subtelocentric as proposed by Pereyra (1992a) may result from the same evolutionary phenomena (i.e., they can be redundant characters), and because there is no banding studies at hand (as were suggested by Borowik, 1995), homology for these characters should be assumed with caution.
The numbers of characters follow the order in the matrix and are separated according to the different sources of data. Apomorphies of genera were coded as binary characters. Abbreviations are as follows: BP (binary polymorphic), BIN (binary non-polymorphic), CON (continuous), MUL (multistate), and MR (majority rule).
I CHARACTERS LIST:
APOMORPHIES FOR LIOLAEMID GENERA (Etheridge, 1995)
Secondary coracoid fenestra present.
Prefrontals wider than long.
Tail spiny.
POTENTIAL APOMORPHIES FOR PHYMATURUS SPECIES GROUPS (Etheridge, 1995)
ALLOZYMES
Characters 28 to 46 are the allozyme described by Pereyra (1992a).
CHROMOSOMES (Pereyra, 1992a)
Number of microchromosomes in females. (0 = 16 microchromosomes; 1 = 18).
EXTERNAL MORPHOLOGY
Number of dorsal scales in head length (CON).
Number of scales in contact with interparietal (CON).
Number of superciliaries (CON).
Subocular fragmentation (number of subocular scales) (CON). Figure 9.
Snout-vent length largest male/largest female ratio (CON).
Surface of dorsal head scales. 0 = smooth; 1 = rugose (BP).
Nasal-rostral contact. 0 = no contact; 1 = contact (BP).
Enlarged scales on posterior gular fold. 0 = absent; 1 = present (BP).
Abdominal region. 0 = immaculate; 1 = spotted (BP).
Head and neck melanism (0 = absent; 1 = present) BIN.
Transverse fine stripes on the back. 0 = absent; 1 = present. BIN. Figure 12.
SKELETAL CHARACTERS
Number of pterygoid teeth (CON).
Number of modified maxillary teeth (heterodonty). Anterior maxillary teeth become unicusped in many taxa (CON).
Medial width of clavicle/skull length. Taken at the point of its flexure (CON).
Anterior process of aritenoid cartilage (0 = at the level of the anterior process of cricoid cartilage; 1 = behind this level) (BP).
Premaxillary teeth (0 = unicusped; 1 = tricusped) (BP).
Cervical rib III (0 = present; 1 = absent) (BP).
Cartilaginous tip of rib IV (0 = bifurcated; 1 = not bifurcated) (BP).
- Barbour, T. 1921. On a small collection of reptiles from Argentina. Proceedings of the Biological Society of Washington, 34:139141.
- Bell, T. 1843. The Zoology of the voyage of HMS "Beagle", under the command of captain Fitzroy, R.N. during the years 1832 to 1836. Edited and superintended by Charles Darwin naturalist to the expedition. Part V: Reptiles. London: Smith (18421843), vi?51 pp. Reprint of the Society for the Study of Amphibians and Reptiles, 1975.
- Borowik, O.A. 1995. Coding chromosomal data for phylogenetic analysis: phylogenetic resolution of the Pan-Homo-Gorilla trichotomy. Systematic Biology, 44:563570.
- Cei, J.M. 1980. New Iguanid Lizards From The Famatina Mountains of Western Argentina. Instituto Biologia Animal, Universidad Nacional de Cuyo, Mendoza, Argentina. Journal of Herpetology, 14:5764.
- Cei, J.M. 1986. Reptiles del centro, centro-oeste y sur de la Argentina. Herpetofauna de las zonas aridas y semiaridas. Monografie IV. Museo Regionale di Historia Naturali di Torino.
- Cei, J.M. 1993. Reptiles del noroeste, nordeste y este de la Argentina. Monografie XIV. Museo Regionale di Historia Naturali di Torino.
- Cei, J.M. & Castro, L.P. 1973. Taxonomic and serological researches on the Phymaturus patagonicus complex. Journal of Herpetology, 7:237247.
- Cei, J.M. & Lescure, J. 1985. Identité de Lacerta palluma Molina, 1782, et revalidation de Centrura flagellifer Bell, 1843 (Reptilia, Sauria). Bulletin du Museum National d'Histoire Naturelle, Paris, Zoologie Biologie et Écologie Animales, 4. Serie, 7(2):451459.
- Cei, J.M. & Videla, F. 2003. A new Phymaturus species from volcanic cordilleran mountains of the south-western Mendoza province, Argentina (Liolaemidae, Iguania, Lacertilia, Reptilia). Bolletino del Museo Regionale di Scienze Naturale, Torino, 20:291314.
- Cei, J.M.; Etheridge, R. & Videla, F. 1983. Especies nuevas de iguánidos del noroeste de la provincia de San Juan (Reserva provincial San Guillermo), Argentina. Deserta, Mendoza, 7:316323.
- Donoso Barros, R. 1964. Reptiles de Chile. Ed. Universidad de Chile, Santiago.
- Espinoza, R.E.; Wiens, J.J. & Tracy, C.R. 2004. Recurrent evolution of herbivory in small,cold-climate lizards: Breaking the ecophysiological rules of reptilian herbivory. PNAS, 101(48):1681916824.
- Etheridge, R.E. 1995. Redescription of Ctenoblepharys adspersa Tschudi, 1845, and the taxonomy of Liolaeminae (Reptilia: Squamata: Tropiduridae). American Museum Novitates, (3142):134.
- Etheridge, R.E. & Espinoza, R.E. 2000. Taxonomy of the Liolaeminae (Squamata: Iguania: Tropiduridae) and a semi-annotated bibliograpy. Smithsonian Herpetological Information Service, 126:164.
- Etheridge, R. & Savage, J.M. 2003. Phymaturus Gravenhorst, 1837 and Lacerta palluma Molina, 1782 (currently Phymaturus palluma; Reptilia, Sauria): proposed conservation of usage of the names by designation of a neotype for Lacerta palluma Molina, 1782. Bulletin of Zoological Nomenclature, 60:3841.
- Farris, J.S. 1970. Methods for computing Wagner trees. Systematics Zoology, 19:8392.
- Flores, G. & Roig-Juñent, S. 2001. Cladistic and biogeographic analysis of the Neotropical genus Epipedonota Solier (Coleoptera: Tenebrionidae), with conservation considerations. Journal of New York Entomological Society, 109:309336.
- Frost, D.R. & Etheridge, R. 1989. A phylogenetic analysis and taxonomy of Iguanian lizards (Reptilia: Squamata). Miscellaneous Publications. Museum of Natural History, University of Kansas, (81):165.
- Goloboff, P.; Farris, J. & Nixon, K. 2003. T.N.T. Tree Analysis Using New Technology. Available at: http://www.zmuc.dk/public/phylogeny
- Hillis, D. 1998. Taxonomic sampling, phylogenetic accuracy, and investigator bias. Systematic Biology, 47:38.
- ICZN. 1999. International Code of Zoological Nomenclature. Fourth Edition. The International Trust for Zoological Nomenclature, London.
- Koslowsky, J. 1898. Enumeración sistemática y distribución geográfica de los reptiles argentinos. Revista del Museo de La Plata, 8:161200.
- Lamborot, M. & Navarro-suárez, M. 1984. Karyotypes and sex determination in Phymaturus palluma palluma Molina (Iguanidae). Herpetologica, 40(3):258264.
- Laurent, R.F. 1984. Tres especies nuevas del género Liolaemus (Reptilia, Iguanidae). Acta Zoologica Lilloana, 37:273299.
- Lescure, J. & Cei, J.M. 1991. L'espece-type du genre Phymaturus Gravenhorst, 1838 (Reptilia, Sauria). Bolletino del Museo Regionale di Scienze Naturale, Torino, 9(1):173175.
- Lobo, F. Las relaciones filogenéticas dentro del grupo chiliensis. (Iguania: Liolaemidae: Liolaemus): sumando nuevos caracteres y taxones. Acta Zoológica Lilloana. In press.
- Lobo, F. 2001. A phylogenetic analysis of lizards of the Liolaemus chiliensis group (Iguania: Tropiduridae). Herpetological Journal, 11:137150.
- Lobo, F. & Abdala, C.S. 2001. Variación morfológica en el esqueleto de Liolaemus (Iguania: Liolaemidae). Búsqueda y descripción de caracteres. Cuadernos de Herpetología, 15:119135.
- Lobo, F. & Abdala, C.S. 2002. La información cladística de un set de datos morfológicos en lagartos del género Liolaemus (Iguania: Liolaemidae). Cuadernos de Herpetología, 16:137150.
- Lobo, F. & Quinteros, S. Taxonomic studies of the genus Phymaturus (Iguania: Liolaemidae): Redescription of Phymaturus patagonicus Koslowsky 1898, and Revalidation and Redescription of Phymaturus spurcus Barbour 1921. Jounal of Herpetology. In press.
- Martínez Carretero, E. 1995. La Puna Argentina: delimitación general y división en distritos florísticos. Boletin de la Sociedad Argentina de Botánica, 31:2740.
- Molina, G.I. 1782. Saggio sulla Storia Naturale del Chile. Stamperia di S. Tommaso d'Aquino, Bologne.
- Morando, M.; Guerreiro, A.C. & Avila, L.J. 2001. Estudios citogenéticos en lagartos del género Phymaturus (Iguanidae: Tropidurinae): cariotipo y mecanismos de determinación sexual en poblaciones del noroeste patagónico. In: Congreso Argentino de Herpetología, 4. Resúmenes. Salta. p.67.
- Morrone, J.J. 1994. Distributional patterns of species of Rhytirrhinini (Coleoptera: Curculionidae) and the historical relationships of the andean provinces. Global Ecology and Biogeography Letters, 4:188194.
- Morrone, J.J. 1996. The biogeographical Andean subregion: a proposal exemplified by arthropod taxa (Arachnida, Crustacea, and Hexapoda). Neotropica, 42:104114.
- Page, R.D. 1994. Maps between trees and cladistic analysis of historical associations among genes, organisms, and areas. Systematic Biology, 43:5877.
- Page, R.D. & Charleston, M.A. 1998. Trees within trees: phylogeny and historical associations. Trends Ecology and Evolution, 13:356359.
- Pereyra, E.A. 1985. Nuevo iguánido del género Phymaturus del noroeste argentino. Boletín de la Asociación Herpetológica Argentina, 2:4.
- Pereyra, E.A. 1991. Phymaturus antofagastensis (Pereyra, 1985) (Tropiduridae, Liolaeminae): ampliación descriptiva. Boletín de la Asociación Herpetológica Argentina, 7:2427.
- Pereyra, E.A. 1992a. Sistemática y relaciones evolutivas de las especies de Phymaturus Gravenhorst, 1838 (Sauria-Liolaeminae). Thesis of Magister, Universidad de Chile. Unpubl.
- Pereyra, E.A. 1992b. Nuevo Tropiduridae del centro-oeste de la Argentina: Phymaturus adrianae n. sp. (Sauria-Liolaeminae). In: Congreso Argentino de Herpetología, 2. Resúmenes. Salta.
- Peters, J.A. & Donoso Barros, R. 1970. Catalogue of the Neotropical Squamata: Part II. Lizards and Amphisbaenians. Bulletin of the United States National Museum, 297:1293.
- Roig-Juñent, S. & Flores, G.E. 2001. Historia biogeográfica de las áreas áridas de América del Sur austral.. In: Llorente Bousquets, J. & Morrone, J.J. (Eds.), Introducción a la biogeografía en Latinoamérica: Teorías, conceptos, métodos y aplicaciones. Las Prensas de Ciencias, Facultad de Ciencias. UNAM, México, D.F. p. 257266.
- Roig-Juñent, S.; Crisci, J.V.; Posadas, P. & Lagos, S. 2002. Areas de distribución y endemismo en zonas continentales. In: Costa, C.; Vanin, S.A.; Lobo, J.M. & Melic, A. (Eds.), Proyecto de Red Iberoamericana de Biogeografía y Entomología Sistemática PRIBES 2002. v.2. Sociedad Entomologica Aragoneza, Zaragoza. p.247266.
- Thiele, K. 1993. The holy grail of the perfect character: the cladistic treatment of morphometric data. Cladistics, 9:275304.
- Schulte, J.A.; Valladares, J.P. & Larson, A. 2003. Phylogenetic relationships within Iguanidae inferred using molecular and morphological data and a phylogenetic taxonomy of iguanian lizards. Herpetologica, 59:399419.
- Scolaro, J. & Cei, J.M. 2003. Una excepcional nueva especie de Phymaturus de la precordillera de Chubut, Argentina (Liolaemidae, Iguania, Lacertilia, Reptilia). Facena, 19:107112.
- Wassersug, R.J. 1976. A procedure for differential staining of cartilage and bone in whole formalin fixed vertebrates. Stain Technology, 51:131134.
- Wiens, J.J. 2000. Coding morphological variation within species and higher taxa for phylogenetic analysis. In: Wiens, J. (Ed.), Phylogenetic analysis of morphological data. Smithsonian Institution Press, Washington. p.115145.
APPENDIX I
APPENDIX II
Publication Dates
-
Publication in this collection
29 Nov 2005 -
Date of issue
2005
History
-
Received
10 Jan 2005 -
Accepted
29 Aug 2005