ABSTRACT
Background: Diffuse axonal injury occurs with high acceleration and deceleration forces in traumatic brain injury (TBI). This lesion leads to disarrangement of the neuronal network, which can result in some degree of deficiency. The Extended Glasgow Outcome Scale (GOS-E) is the primary outcome instrument for the evaluation of TBI victims. Diffusion tensor imaging (DTI) assesses white matter (WM) microstructure based on the displacement distribution of water molecules.
Objective: To investigate WM microstructure within the first year after TBI using DTI, the patient’s clinical outcomes, and associations.
Methods: We scanned 20 moderate and severe TBI victims at 2 months and 1 year after the event. Imaging processing was done with the FMRIB software library; we used the tract-based spatial statistics software yielding fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) for statistical analyses. We computed the average difference between the two measures across subjects and performed a one-sample t-test and threshold-free cluster enhancement, using a corrected p-value < 0.05. Clinical outcomes were evaluated with the GOS-E. We tested for associations between outcome measures and significant mean FA clusters.
Results: Significant clusters of altered FA were identified anatomically using the JHU WM atlas. We found increasing spotted areas of FA with time in the right brain hemisphere and left cerebellum. Extensive regions of increased MD, RD, and AD were observed. Patients presented an excellent overall recovery.
Conclusions: There were no associations between FA and outcome scores, but we cannot exclude the existence of a small to moderate association.
Keywords: Craniocerebral Trauma; Diffuse Axonal Injury; Diffusion Tensor Imaging; Glasgow Outcome Scale; Regeneration
RESUMO
Antecedentes: A lesão axonial difusa ocorre em traumas com alta energia de aceleração e desaceleração, determinando desorganização da microestrutura cerebral, levando a algum déficit. A escala de Glasgow Estendida (GOS-E) é indicada na avaliação clínica das vítimas de trauma cranioencefalico. Imagens por tensor de difusão (DTI) estudam a microestrutura cerebral a partir da difusão das moléculas de água.
Objetivo: Investigar a microestrutura cerebral no primeiro ano após trauma, avaliar clinicamente os pacientes e testar para correlações entre estes resultados.
Métodos: 20 vítimas de TCE moderado e grave foram avaliados 2 meses e 1 ano depois do trauma. O processamento foi feito usando o software FMRIB (FSL) e a análise estatística foi feita com tract-based spatial statistics software para extrair os parâmetros de DTI. Calculamos a diferença da média entre as duas observações de cada sujeito e fizemos um teste-t para uma amostra e threshold-free cluster enhancement. Realizamos correções para múltiplas comparações e determinamos o valor de p < 0.05 como significativo. Um ano após o trauma, a avaliação clínica foi feita usando a GOS-E. Testamos para associações entre os resultados clínicos e os valores médios de FA dos clusters.
Resultados: Os clusters significativos foram identificados usando o atlas JHU WM. Observamos aumento de FA predominantemente no hemisfério cerebral direito e cerebelar à esquerda e também extensas áreas de aumento nos demais parâmetros de DTI. A recuperação dos pacientes foi satisfatória.
Conclusões: Não encontramos associações entre os resultados, no entanto alguma associação pequena a moderada não pode ser excluída.
Palavras-chave: Traumatismos Craniocerebrais; Lesão Axonal Difusa; Imagem de Tensor de Difusão; Escala de Resultado de Glasgow; Regeneração
INTRODUCTION
Traumatic brain injury (TBI) causes different complex brain lesions such as hematomas, contusions, vascular injuries, and diffuse axonal injury (DAI). DAI results from high-energy acceleration and deceleration forces, determining shearing strains in the white matter, leading to disconnection or dysfunction of the neural network1.
Head injuries, particularly DAI, result in distinct functional deficits, such as physical, cognitive, and behavioral impairments, which dramatically affect life quality, return to daily activities, and social reintegration of survivors2. In 1975, Jennett and Bond developed the Glasgow Outcome Scale (GOS), and it was used as a primary outcome measure in phase III trials in TBI3,4. Afterward, acknowledging some limitations of the GOS, the Glasgow Outcome Scale - Extended (GOS-E) was developed. Since its establishment in 1981, it has been used and recommended as the primary outcome measurement in TBI studies5,6.
DAI is not only restricted to mechanical forces at the moment of the trauma. Many different processes are triggered, such as inflammatory responses, molecular changes, apoptosis, and Wallerian degeneration. Therefore, the pathophysiology of DAI can be divided into primary and secondary lesions. The primary axonal lesion is the complete disconnection related to the kinetic energy in the moment of trauma. In contrast, secondary axonal injuries are indirect and progressive lesions in neurons that occur late after the initial shock7. The impact sparks molecular and cellular events that disturb the homeostasis, leading to changes in neurons and to the regional microglia that can persist for years8.
Traditional imaging modalities such as computed tomography and standard magnetic resonance (MR) sequences, such as T1 and T2 weighted sequences, are not sensible enough to show the white matter (WM) damage related to DAI. Diffusion tensor imaging (DTI) is an advanced MR modality based on water molecules diffusion that measures the preferential displacement along the white matter tracts and has been used to assess the brain microstructure in different pathologies, including head injuries9. There are diverse methods available to analyze DTI images, such as region-of-interest analysis and tractography. One of the most commonly used is the whole-brain approach for group comparisons, for which tract-based spatial statistics (TBSS) is particularly recommended for voxel wise and cluster-based analyses, constraining statistical analysis to the center of the tracts9. It is a semi-automated method, with minimal user-dependence, that allows a whole-brain evaluation and is notably suitable for evaluating diffuse lesions in the brain parenchyma such as DAI10,11.
Other groups have used this approach to assess white matter changes in victims of head injury in different stages after trauma12,13. Lipton and colleagues conducted a study on patients with mild TBI who presented with persistent cognitive impairment eight months to three years after the trauma. They found decreased fractional anisotropy (FA) and increased mean diffusivity (MD) in the corpus callosum, subcortical white matter, and internal capsules compared to healthy controls13. Another group investigated adolescents with mild TBI in the acute phase (from 1 to 6 days after the trauma event) compared to age-matched controls14. They found significantly decreased apparent diffusion coefficient (ADC) and radial diffusivity (RD) and increased FA in several white matter regions and the left thalamus, consistent with axonal cytotoxic edema in the acute phase post-injury. However, few published works analyzed the progressive changes in the white matter in DAI, particularly in moderate and severe trauma victims.
This study aimed to investigate longitudinally the white matter of patients with severe and moderate DAI at two moments defined as the subacute (two months) and early chronic phases (one year) following the trauma event. We also assessed patients’ clinical outcome one year after trauma using the GOS-E scale6. Our central hypothesis is that DTI parameters change with time and can have a degree of correlation with functional outcome.
METHODS
Standard protocol approvals
The protocol was reviewed and approved by the institutional review board, the local ethics committee, and all participants gave written informed consent.
Study design and subjects
A prospective study was conducted throughout one year. Adult outpatients admitted at the Emergency Room of Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, Brazil, victims of moderate and severe TBI (Glasgow Coma Scale scores between 3 and 12 at initial evaluation), presenting clinical and tomographic findings exclusively of DAI were eligible to be included in the study. Exclusion criteria were the presence of contusions greater than 10 cm3, midline shift greater than 0.5 cm, extra-axial collection determining compression of the brain parenchyma, or any indication for surgical intervention. Patients with poor quality imaging studies that limited analysis, clinical contra-indications that precluded MR scanning, or loss of follow-up were also excluded.
Data acquisition
All data were acquired on a 3T system (Intera Achieva, Philips Healthcare, Best, The Netherlands). Patients were scanned using an 8-channel head proton coil (Philips Healthcare, Best, The Netherlands) at two time-points: two months (subacute phase) and one year (early chronic phase) after the trauma. The routine protocol included fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), and susceptibility-weighted imaging (SWI) sequences. For the data analysis in this study, we used a volumetric T1-weighted and DTI sequences.
The 3D-T1 fast field echo, acquired in the sagittal plane, was obtained using the following parameters: FOV 240 x 240 x 180 mm3; matrix 240 x 240 mm; isotropic resolution; TR/TE 6.2/2.7 ms; and acquisition time 4.13 min.
The DTI sequence was acquired in the axial plane, using 32 directions and one b0 using the following parameters: 70 slices; slice thickness 2 mm; no gap; field of view 256 x 256 mm; voxel resolution = 2 mm3 (isotropic); TR/TE 8.500/61 ms; b = 1000 s/mm2; matrix 128 x 128; number of excitations (NEX) = 1; and acquisition time of 7 minutes.
Imaging processing and analysis
Initially, all diffusion images were pre-processed for eddy current corrections and extraction of non-brain voxels, using FMRIB's Diffusion Toolbox (FSL) software, version 5.0.119,15. For motion correction, the free toolbox Explore DTI (A. Leemans, University Medical Center, Utrecht, The Netherlands) was used, which rotates the B-matrix while keeping the exact initial orientation. With this same software, visual quality inspection for residuals and outliers was performed in each data set16,17.
Thereafter, FA maps were analyzed using TBSS9. All individual FA images were non-linearly registered to the most typical subject of the sample (using -n command), and then the aligned dataset was transformed into the MNI152 standard space (1 mm3). The mean aligned FA images were merged into a single four-dimensional (4D) average FA image. A mean FA skeleton was extracted from the generalized 4D image, and the tracts were projected into the skeleton, using a 0.2 threshold18. To extract mean, axial, and radial diffusivities (MD, AD, and RD, respectively), non-linear warps and skeleton projections were applied to each DTI scalar parameter.
Statistical analysis
To assess differences in FA, MD, RD, and AD with time, we performed one-sample t-tests, using the average difference between the two measures across subjects. Initially, the difference between the subacute and the early chronic phase was calculated, and then the early chronic value minus the subacute phase value was calculated. Permutation-based nonparametric inferences were made on unsmoothed statistical maps, using 5000 permutations, and the cluster-like structures were enhanced using the threshold-free cluster enhancement (TFCE) algorithm19. This approach was similarly applied to the MD, AD, and RD maps. Data were corrected for multiple comparisons, using the family-wise error (FWE) rate, setting the significance level at p < 0.05.
Thenceforth, the cluster tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Cluster) was applied to extract the exact clusters, followed by the Atlasquery tool to obtain the coordinates (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlasquery) according to the Johns Hopkins University (JHU) white matter tractography atlas.
Outcome measure
We used the GOS-E at 12 months post-injury obtained at the medical appointment follow-up, which has been recommended as the main outcome measurement in TBI studies7. It consists in an eight-scale global measure of function, used to estimate physical disability grading6. It classifies patients into upper and lower levels of good recovery (GOS-E = 7 and 8), moderate disability (GOS-E = 5 and 6), severe disability (GOS-E = 3 and 4), vegetative state (GOS-E =2) and death (GOS-E =1).
Association analysis
The WM areas with FA differences with time were defined as ROIs and the mean FA values of each one was calculated. Then, to test for association of mean FA values of each ROI with GOS-E grading, we used Cohen’s d effect size test. We segmented patients into two different groups: sub-optimal (GOS-E= 5 or 6) and optimal (GOS-E = 7 or 8) performance. We tested for associations of each ROI at two months and one year after trauma.
Taking into account the relatively small patient sample, we also estimated Cohen’s d effect size test considering a bigger sample size (4 times our sample, with the same distribution).
RESULTS
In the initial screening, 225 patients with head trauma were evaluated, and the final analysis included twenty of those patients. Demographics of the final sample are described in Table 1. Two hundred and five subjects were excluded for the following reasons:
-
- 186 had no clinical and/or tomographic criteria for DAI;
-
- 7 follow-up losses;
-
- 5 were not eligible for MRI;
-
- 5 had low-quality DTI studies;
-
- 1 developed epidural compressive hematoma;
-
- 1 died.
Evaluation of changes between two months and one year after trauma (chronic minus subacute volumes) with voxel-based TFCE analysis indicated brain regions with FA increment with time, predominantly in the right hemisphere and in the left cerebellum. Significant brain clusters (Table 2) were found in the right superior longitudinal fascicle, the temporal part of the right superior longitudinal fascicle, right inferior fronto-occipital fascicle, right superior and inferior longitudinal fascicles, the body of corpus callosum, forceps major and left corticospinal tract (Figure 1). Moreover, we found extensive areas of increases in MD, RD, and AD (p < 0.05, FWE corrected) (Figure 2).
The most significant clusters found with increments in FA (early chronic phase minus subacute phase) are shown in red, TFCE (p < 0.05, FWE corrected). The mean FA skeleton is indicated in white.
White matter differences between early chronic and subacute phases. Significant clusters (p < 0.05, FWE corrected). Blue depicts MD, yellow AD, and green RD increases in the chronic phase.
Of note, the one-sample t-test used to assess the difference between subacute and early chronic volumes did not demonstrate significant differences for any DTI parameter.
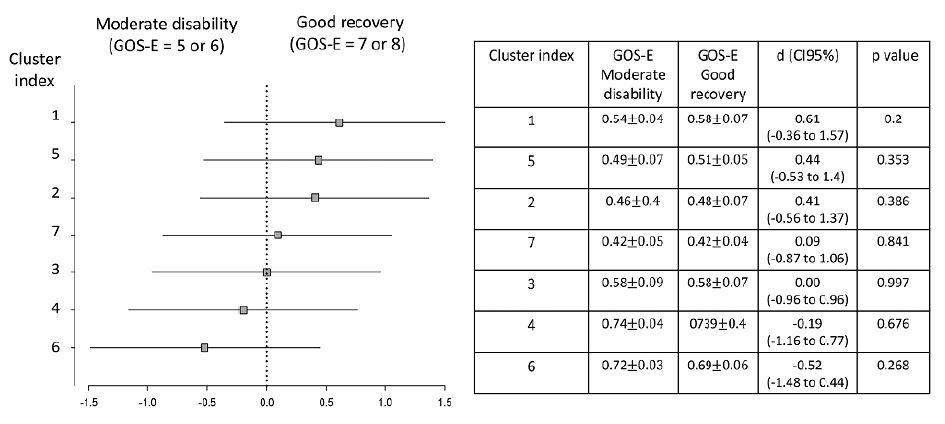
Correlations between the different FA ROIs and the one-year GOS-E grades were tested with different ROIs at 2 months and 1 year post-trauma (Figures 3 and 4). We did not find any correlations on either moment.
Subgroup analysis. The forest plot shows the effect size in the outcome variable across the pre-specified subgroups according to GOS-E outcome stratification (moderate disability vs good recovery). Association analysis between different ROIs at 2 months after trauma with sub-optimal and optimal 1-year post-trauma GOS-E scores. Horizontal axis indicates differences between the groups of recovery according to each cluster. Effect size values are displayed with respective 95% confidence intervals and statistical significance (p) obtained by Cohen’s d test (squares).
Subgroup analysis. The forest plot shows the effect size in the outcome variable across the pre-specified subgroups according to GOS-E outcome stratification (moderate disability vs good recovery). Association analysis between different ROIs at one year after trauma with sub-optimal and optimal 1-year post-trauma GOS-E scores. Horizontal axis indicates differences between groups of recovery according to each cluster. Effect size values are shown with respective 95% confidence intervals and statical significance (p) obtained by Cohen’s d test.
In addition, by hypothetically increasing our sample 4-fold, we found some associations between one-year GOS-E and the specific ROIs of FA increase at 2 months and 1 year after trauma (Table 3).
DISCUSSION
In our investigation, we performed whole-brain analysis using a semi-automated method to explore white matter changes over time in moderate and severe TBI victims. DTI has mainly been used to study white matter in the trauma scenario. However, most published articles are related to mild trauma and with different follow-up periods12,13,20. It is important to emphasize that our patient sample is very homogeneous, consisting of victims with moderate and severe trauma, who were explicitly and exclusively diagnosed with DAI, and followed for one year after the event.
We found some scattered areas of FA increase, notably in the right brain hemisphere, accompanied by vast regions in the brain and the cerebellum demonstrating an increase in MD, RD, and AD over time. Interestingly, patients showed relatively good clinical outcomes, according to the GOS-E scale. We also found different associations between each brain region with increased FA and the late clinical outcome (GOS-E) two months and one year after trauma, which were more prominent when tested in a larger sample size. Our results are aligned with previous studies that have described white matter changes on DTI parameters over with time in victims of head trauma21,22. These ongoing DTI parameters are related to different pathophysiological processes such as inflammation, degeneration, and regeneration - which have already been described in experimental studies23,24.
We identified a general area of increase in MD, AD, and RD in brain tracts one year after trauma. We consider that the MD increase is mainly a result of high RD values and, in a lower degree, to AD increment. MD represents the overall diffusivity of water molecules, which can be related to the increasing content of isotropic tissue with water content (gliosis)25. Although the biological basis for anisotropy and diffusivity changes in tissues revealed by DTI data is still largely debated, studies using animal models have demonstrated that axonal injury itself is represented by AD changes, and demyelination is associated with an increase in RD values26. Considering that increases in both AD and RD contribute positively to increase in MD values, it is reasonable to assume MD as a more sensitive parameter when compared to FA in our observation.
Moreover, in addition to axonal injury, other important and specific pathophysiological processes are also present in the trauma scenario, such as neuroinflammation, afferent degeneration, and debris clearance, and the magnitude of each one at different stages may imply distinct changes in DTI scalar values. Animal model studies play an essential role in characterizing these other effects of the trauma event and how they change over time. However, most of the articles published to date describe the changes that occur in the early acute time after trauma, and only a limited number of articles evaluate long-term consequences27. It is already well established that the overall axonal injury in trauma survivors is a consequence of the secondary axonal injury, which is the indirect damage to neurons related to neuroinflammation and microglial activation, triggered by the initial impact and that can persist for years23. These processes are responsible for biochemical changes leading to local edema and changes in the microvascular circulation, leading to ischemia and demyelination, which can be confirmed by the RD increase over time28. Moreover, AD increase has been associated with an increase of the extracellular water content, such as debris clearance, that would ease the water molecule movement in an axis parallel to the axons29. Thereby, we suppose that our results can be explained by the Wallerian or Wallerian-like degeneration process due to DAI or related to a secondary pathological process, such as regional ischemia, and neuronal death may ultimately lead to brain atrophy29.
We also found some spotted areas of FA increase in the right brain hemisphere and the left cerebellum over time. Different causes can be associated with FA increase, such as local fibrosis, hemorrhage areas, and neuronal sprouting30. FA is related to the microstructural organization, with high values (close to one) related to most anisotropic tissues. Microstructural organization after trauma has been reported to start in the first few days and can persist for years, which is linked to neuroplasticity31. The functional recovery accompanied by the increase in FA may somehow be related to neuroplasticity. Interestingly, we found areas of FA increase in the right brain hemisphere and in the left cerebellum, which may indicate the involvement of the contralateral cerebellar hemisphere in functional and compensatory changes after trauma, as it has been already reported32. An interesting functional study compared children with moderate and severe trauma to controls, showing that children with TBI demonstrated changes in functional cerebral activity and increased recruitment of neural resources such as the cerebellum32.
We tested for correlations between mean FA values at the subacute and early chronic phases of the specific regions that presented significant changes over time and the GOS-E scores. We could not find any significant correlations, but the lack of significance may be related to our sample size, which was relatively small when considering the optimal number of individuals required for correlational studies33. Still, some specific regions, such as the right SLF and the body of the corpus callosum, demonstrated promising effect sizes in functional stratification at the early chronic phase between optimal and sub-optimal GOS-E scores and mean FA values by using a theoretical larger sample size.
Whole-brain voxel-wise analysis has been increasingly used to study DAI because of the widespread nature of the disorder and the advantage of this method being minimally invasive for multi-subject group evaluation. However, with this technique, it is imperative the use rigorous statistical procedures to correct for multiple comparison errors, which reduce the sensitivity for detecting subtle changes12.
One limitation of our study is the relatively small sample size. However, we included an homogeneous group of patients with a minimum one-year survival after the traumatic event, especially considering that victims of moderate and severe head trauma have high mortality rates in the first six months34,35. Moreover, these patients also presented an excellent recovery with high one-year GOS-E scores. This may be related to the exclusion of other conditions commonly associated with a head injury, such as contusions and hematomas that are related to a worse outcome2.
Concerning the methodology and image acquisition, we must emphasize that more gradient encoding directions and more robust DTI acquisition and analytical methods such as high angular resolution diffusion imaging (HARDI), diffusion kurtosis imaging (DKI), and q-ball imaging are available and could have enhanced the power of data analysis11,36. However, these approaches require longer acquisition times, more sophisticated algorithms, and are still not feasible to implement in clinical and research scenarios.
In conclusion, our work indicated changes in all DTI scalar metrics in the brain and cerebellum white matter in a homogeneous group of DAI victims along the first year following moderate and severe head trauma. This study can be important to guide future research in understanding the different pathophysiological processes that occur at different stages of patient recovery. Further studies are expected to show that DTI is a tool for signaling functional outcomes and is a promising method to guide therapies and rehabilitation procedures in trauma survivors.
ACKNOWLEDGMENTS
We are thankful to FAPESP (Sao Paulo Research Foundation) for financial support. Grants 2015/18136-1, 2016/05547-6, 2017/17065-9.
REFERENCES
-
1. Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989 Jul;15(1):49-59. https://doi.org/10.1111/j.1365-2559.1989.tb03040.x
» https://doi.org/10.1111/j.1365-2559.1989.tb03040.x -
2. Esbjörnsson E, Skoglund T, Sunnerhagen KS. Fatigue, psychosocial adaptation and quality of life one year after traumatic brain injury and suspected traumatic axonal injury; evaluations of patients and relatives: a pilot study. J Rehabil Med. 2013 Sep;45(8):771-7. https://doi.org/10.2340/16501977-1170
» https://doi.org/10.2340/16501977-1170 -
3. Vieira RCA, Paiva WS, de Oliveira DV, Teixeira MJ, de Andrade AF, de Sousa RMC. Diffuse axonal injury: epidemiology, outcome and associated risk factors. Front Neurol. 2016 Oct 20;7:178. https://doi.org/10.3389/fneur.2016.00178
» https://doi.org/10.3389/fneur.2016.00178 -
4. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975 Mar 1;1(7905):480-4. https://doi.org/10.1016/S0140-6736(75)92830-5
» https://doi.org/10.1016/S0140-6736(75)92830-5 -
5. Wilde EA, Whiteneck GG, Bogner J, Bushnik T, Cifu DX, Dikmen S, et al. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch Phys Med Rehabil. 2010 Nov 1;91(11):1650-60.e17. https://doi.org/10.1016/j.apmr.2010.06.033
» https://doi.org/10.1016/j.apmr.2010.06.033 -
6. Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981 Apr 1;44(4):285-93. https://doi.org/10.1136/jnnp.44.4.285
» https://doi.org/10.1136/jnnp.44.4.285 -
7. Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013 Aug;246:35-43. https://doi.org/10.1016/j.expneurol.2012.01.013
» https://doi.org/10.1016/j.expneurol.2012.01.013 -
8. Bramlett HM, Dietrich WD. Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma. 2015 Dec 1;32(23):1834-48. https://doi.org/10.1089/neu.2014.3352
» https://doi.org/10.1089/neu.2014.3352 -
9. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-19. https://doi.org/10.1016/j.neuroimage.2004.07.051
» https://doi.org/10.1016/j.neuroimage.2004.07.051 -
10. Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol. 2013 Nov-Dec;34(11):2064-74. https://doi.org/10.3174/ajnr.A3395
» https://doi.org/10.3174/ajnr.A3395 -
11. Su E, Bell M. Diffuse axonal injury. In: Laskowitz D, Grant G, editors. Translational research in traumatic brain injury. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016. Chapter 3. Available from: https://www.ncbi.nlm.nih.gov/books/NBK326722/
» https://www.ncbi.nlm.nih.gov/books/NBK326722/ -
12. Garin-Muga A, Borro D. Review and challenges of brain analysis through DTI measurements. Stud Health Technol Inform. 2014;207:27-36. https://doi.org/10.3233/978-1-61499-474-9-27
» https://doi.org/10.3233/978-1-61499-474-9-27 -
13. Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disbility: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma. 2008 Nov;25(11):1335-42. https://doi.org/10.1089/neu.2008.0547
» https://doi.org/10.1089/neu.2008.0547 -
14. Chu Z, Wilde EA, Hunter JV, Mccauley SR, Bigler ED, Troyanskaya M, et al. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am J Neuroradiol. 2010 Feb;31(2):340-6. https://doi.org/10.3174/ajnr.A1806
» https://doi.org/10.3174/ajnr.A1806 -
15. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001 Jun;5(2):143-56. https://doi.org/10.1016/s1361-8415(01)00036-6
» https://doi.org/10.1016/s1361-8415(01)00036-6 -
16. Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 2009 Jun;61(6):1336-49. https://doi.org/10.1002/mrm.21890
» https://doi.org/10.1002/mrm.21890 - 17. Leemans A, Jeurissen B, Sijbers J, Jones DK. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc Int Soc Magn Reson Med. 2009 Jan;17(2):3537.
-
18. Smith SM, Jenkinson M, Johansen-berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006 Jul 15;31(4):1487-505. https://doi.org/10.1016/j.neuroimage.2006.02.024
» https://doi.org/10.1016/j.neuroimage.2006.02.024 -
19. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009 Jan 1;44(1):83-98. https://doi.org/10.1016/j.neuroimage.2008.03.061
» https://doi.org/10.1016/j.neuroimage.2008.03.061 -
20. Wallace EJ, Mathias JL, Ward L. Relationship between diffusion tensor imaging findings and cognitive outcomes following adult traumatic brain injury: a meta-analysis. Neurosci Biobehav Rev. 2018 Sep;92:93-103. https://doi.org/10.1016/j.neubiorev.2018.05.023
» https://doi.org/10.1016/j.neubiorev.2018.05.023 -
21. O’Phelan KH, Otoshi CK, Ernst T, Chang L. Common patterns of regional brain injury detectable by diffusion tensor imaging in otherwise normal-appearing white matter in patients with early moderate to severe traumatic brain injury. J Neurotrauma. 2018 Mar 1;35(5):739-49. https://doi.org/10.1089/neu.2016.4944
» https://doi.org/10.1089/neu.2016.4944 -
22. Leon AMC, Cicuendez M, Navarro B, Munarriz PM, Cepeda S, Paredes I, et al. What can we learn from diffusion tensor imaging from a large traumatic brain injury cohort?: white matter integrity and its relationship with outcome. J Neurotrauma. 2018 Oct 15;35(20):2365-76. https://doi.org/10.1089/neu.2018.5691
» https://doi.org/10.1089/neu.2018.5691 -
23. Sullivan GM, Mierzwa AJ, Kijpaisalratana N, Tang H, Wang Y, Song S-K, et al. Oligodendrocyte lineage and subventricular zone response to traumatic axonal injury in the corpus callosum. J Neuropath Exp Neurol. 2013 Dec 1;72(12):1106-25. https://doi.org/10.1097/NEN.0000000000000009
» https://doi.org/10.1097/NEN.0000000000000009 -
24. Tu T-W, Williams RA, Lescher JD, Jikaria N, Turtzo LC, Frank JA. Radiological-pathological correlation of diffusion tensor and magnetization transfer imaging in closed head traumatic brain injury model. Ann Neurol. 2016 Jun;79(6):907-20. https://doi.org/10.1002/ana.24641
» https://doi.org/10.1002/ana.24641 -
25. Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, et al. Water diffusion changes in wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001 Jun;13(6):1174-85. https://doi.org/10.1006/nimg.2001.0765
» https://doi.org/10.1006/nimg.2001.0765 -
26. Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002 Nov;17(3):1429-36. https://doi.org/10.1006/nimg.2002.1267
» https://doi.org/10.1006/nimg.2002.1267 -
27. Osier ND, Carlson SW, DeSana A, Dixon CE. Chronic histopathological and behavioral outcomes of experimental traumatic brain injury in adult male animals. J Neurotrauma. 2015 Dec 1;32(23):1861-82. https://doi.org/10.1089/neu.2014.3680
» https://doi.org/10.1089/neu.2014.3680 -
28. Rubovitch V, Ten-Bosch M, Zohar O, Harrison CR, Tempel-Brami C, Stein E, et al. A mouse model of blast-induced mild traumatic brain injury. Exp Neurol. 2011 Dec;232(2):280-9. https://doi.org/10.1016/j.expneurol.2011.09.018
» https://doi.org/10.1016/j.expneurol.2011.09.018 -
29. Hutchinson EB, Schwerin SC, Avram AV, Juliano SL, Pierpaoli C. Diffusion MRI and the detection of alterations following traumatic brain injury. J Neurosci Res. 2018 Apr;96(4):612-25. https://doi.org/10.1002/jnr.24065
» https://doi.org/10.1002/jnr.24065 -
30. Genc S, Anderson V, Ryan NP, Malpas CB, Catroppa C, Beauchamp M, et al. Recovery of white matter following paediatric traumatic brain injury depends on injury severity. J Neurotrauma. 2017 Feb 15;34(4):798-806. https://doi.org/10.1089/neu.2016.4584
» https://doi.org/10.1089/neu.2016.4584 -
31. Harris NG, Verley DR, Gutman BA, Sutton RL. Bi-directional changes in fractional anisotropy after experiment TBI: disorganization and reorganization? Neuroimage. 2016 Jun;133:129-43. https://doi.org/10.1016/j.neuroimage.2016.03.012
» https://doi.org/10.1016/j.neuroimage.2016.03.012 -
32. Caeyenberghs K, Wenderoth N, Smits-Engelsman BCM, Sunaert S, Swinnen SP. Neural correlates of motor dysfunction in children with traumatic brain injury: exploration of compensatory recruitment patterns. Brain. 2009 Mar;132(3):684-94. https://doi.org/10.1093/brain/awn344
» https://doi.org/10.1093/brain/awn344 -
33. Sullivan GM, Feinn R. Using effect size - or why the P value is not enough. J Grad Med Educ. 2012 Sep;4(3):279-82. https://doi.org/10.4300/JGME-D-12-00156.1
» https://doi.org/10.4300/JGME-D-12-00156.1 -
34. Saatman KE, Duhaime A-C, Bullock R, Maas AIR, Valadka A, Manley GT, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008 Jul;25(7):719-38. https://doi.org/10.1089/neu.2008.0586
» https://doi.org/10.1089/neu.2008.0586 -
35. Brown AW, Leibson CL, Mandrekar J, Ransom JE, Malec JF. Long-term survival after traumatic brain injury: a population-based analysis controlled for nonhead trauma. J Head Trauma Rehabil. 2014 Jan-Feb;29(1):E1-8. https://doi.org/10.1097/HTR.0b013e318280d3e6
» https://doi.org/10.1097/HTR.0b013e318280d3e6 -
36. Grassi DC, Conceição DMD, Leite CC, Andrade CS. Current contribution of diffusion tensor imaging in the evaluation of diffuse axonal injury. Arq Neuropsiquiatr. 2018 Mar;76(3):189-99. https://doi.org/10.1590/0004-282X20180007
» https://doi.org/10.1590/0004-282X20180007
Publication Dates
-
Publication in this collection
18 Mar 2022 -
Date of issue
Mar 2022
History
-
Received
07 Feb 2021 -
Reviewed
17 Apr 2021 -
Accepted
30 May 2021
 Longitudinal whole-brain analysis of multi-subject diffusion data in diffuse axonal injury
Longitudinal whole-brain analysis of multi-subject diffusion data in diffuse axonal injury




 FA: fractional anisotropy.
FA: fractional anisotropy.
 FWE: family-wise error; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity.
FWE: family-wise error; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity.