ABSTRACT:
This study evaluated the sanitary hygienic quality and the presence of pathogenic microorganisms in raw meats and fresh sausages marketed in the city of Uruguaiana, Rio Grande do Sul (RS), Brazil. We analyzed 238 samples of fresh sausages, beef, pork, and chicken from 18 commercial establishments (butchers, supermarkets, and groceries). Samples were subjected to enumerate hygiene indicator microorganisms (mesophilic aerobes and enterobacteria) and detection of Salmonella spp. and Listeria monocytogenes. The mean counts of mesophilic aerobes and enterobacteria were 5.09 and 3.54 log CFU/g, respectively. Beef samples presented the highest frequency of Salmonella spp. (7.93%) and fresh sausages present the highest frequency of L. monocytogenes (19.04%). Among the analyzed samples, 43.70% did not comply with the microbiological parameters established by the Brazilian Ministry of Health. The presence of Salmonella spp. and L. monocytogenes in different samples and commercial establishments demonstrate the failures of good manufacturing practices in industrial environmental and retails points and the need to train food handlers to reduce the exposure of consumers to potential risks.
Key words: microbial contamination; pathogens; retail trade; sanitary hygienic quality
RESUMO:
Este estudo teve como objetivo avaliar a qualidade higiênica sanitária e a presença de microrganismos patogênicos em carnes in natura e linguiças frescais comercializadas na cidade de Uruguaiana, Rio Grande do Sul (RS), Brasil. Foram analisadas 238 amostras de linguiças, carne bovina, suína e de frango de 18 estabelecimentos comerciais (açougues, supermercados e mercearias). As amostras foram submetidas à enumeração de microrganismos indicadores de higiene (aeróbios mesófilos e enterobactérias) e detecção de Salmonella spp. e Listeria monocytogenes. As contagens médias de aeróbios mesófilos e enterobactérias foram 5,09 e 3,54 log UFC/g, respectivamente. Salmonella spp. esteve presente mais frequentemente em amostras de carne bovina (4,91 %), enquanto L. monocytogenes foi mais frequente em linguiças frescais (19,04 %). Das amostras analisadas, 43,70 % não atenderam aos parâmetros microbiológicos estabelecidos pelo Ministério da Saúde. A presença de Salmonella spp. e L. monocytogenes em diferentes amostras e estabelecimentos comerciais demonstra falhas de boas práticas de fabricação na indústria e pontos de venda, além da necessidade de treinar manipuladores de alimentos para reduzir a exposição dos consumidores à riscos.
Palavras-chave: comércio varejista; contaminação microbiana; patógenos; qualidade higiênico-sanitária
INTRODUCTION:
The high consumption of meat and meat products is a trend in developing countries like Brazil and has led to increased concerns from health authorities regarding food quality and safety (LOPES et al., 2017; ABUJAMRA et al., 2017; FANALLI et al., 2018). Raw meats and fresh sausages are characterized by high water content, and the presence of nutrients and pH that provide a favorable environment for multiplication of microorganisms (LAWRIE, 2005). Failures in good manufacturing practices at any stage of the processing can result in contamination by pathogenic microorganisms, demanding rigorous control along the production chain (JARVIS et al. 2016; DEMAÎTRE et al., 2020).
The commercialization and domestic consumption of food are the last stages of the production chain and are considered critical concerning hygienic-sanitary conditions. In Brazil, meat products available for retail must meet the microbiological requirements established by the National Health Surveillance Agency (ANVISA) (BRASIL, 2001; BRASIL, 2019), which establishes acceptance criteria for food. In the same way, these products must meet the conditions of storage and commercialization that are set and monitored by health surveillance agencies located in the municipalities (BRASIL, 1993).
However, many challenges are faced during the sanitary inspection of food retail establishments, especially in cities in the interior and with a low Human Development Index (HDI). RESENDE and SANTOS (2012) reported that in these places, understanding sanitary legislation in public health is a difficult task. This scenario becomes more complicated in border regions, such as the West Frontier region of Rio Grande do Sul (RS), where the city of Uruguaiana is located. The difficulties in understanding and applying health legislation in these regions must be added to the lack of structuring of health surveillance bodies, insufficient technical personnel, distance from animal food centers and the variability of products available to the population (PEREIRA et al., 2017). As a result of this insufficient infrastructure and inspection in markets, butchers and grocery stores, products of animal origin that do not meet the basic hygiene and food handling standards are often commercialized, resulting in contamination and spread of pathogens to the consumer.
The evaluation of indicator and pathogenic microorganisms in establishments selling meat and meat products may identify failures at all points of the production chain, that is, from slaughter and processing at the industrial level to handling at points of sale directly to consumers (SAINI et al., 2011). Salmonella spp. and L. monocytogenes are among the main bacterial pathogens responsible for foodborne disease outbreaks and, due to their ability to tolerate adverse conditions, they can be found in environments where animal and plant foods are produced, processed, and manipulated (CAVALIN et al., 2018; ESFA, 2018; GONÇALVES-TENÓRIO et al., 2018; MOHAMED et al., 2018; CDC, 2019; CUNHA-NETO et al., 2019; ZENG et al., 2019; BRASIL, 2020).
The dissemination of scientific data regarding the presence of indicator and pathogenic microorganisms in foods from this region would focus the population’s attention on microbiological quality and could lead the subsidization of health education programs that will benefit the health of individuals living in these places. Thus, we designed this study to evaluate sanitary hygienic quality through the enumeration of enumeration of mesophilic aerobes and enterobacteria and detection of Salmonella spp. and L. monocytogenes in meat cuts and fresh sausages marketed in the city of Uruguaiana, RS - Brazil, and to evaluate the conformity of these products regarding microbiological parameters established by official control bodies.
MATERIALS AND METHODS:
Sample collection
A total of 238 chilled raw meat and fresh sausage samples were collected from retail stores in Uruguaiana, RS, Brazil, from January 2017 to June 2018. Of these samples, 84 were from fresh sausages, 63 from beef (ground beef), 61 from chicken (breast, wings, and drumsticks), and 30 from pork (chops). These samples were obtained from 18 establishments, including seven butcher shops (n = 97), seven supermarkets (n = 95), and four groceries (n = 46). All products had indications that they were produced in Brazil. Approximately 300 g of various products was purchased and kept in their original packaging at 4 °C, in an isothermal box with recyclable ice, until analyses were performed.
Microbiological analysis
Enumeration of mesophilic aerobes and enterobacteria
Twenty-five grams of each sample were transferred to sterile bags with 225 mL NaCl 0.9% (w/v), homogenized and subjected to ten-fold dilution with NaCl 0.9% (w/v) (BACTERIOLOGICAL ANALYTICAL MANUAL/BAM-USDA, 2003). Then, dilutions were plated for enumeration of mesophilic aerobes (plate count agar, PCA, Oxoid, Hampshire, UK) and enterobacteria (violet red bile glucose agar, VRBGA, Oxoid) and incubated at 36°C for 48 and 24 h, respectively. After incubation, colonies were enumerated and results were presented as log colony-forming units per gram (log CFU/g).
Salmonella spp.
Salmonella spp. were detected with the protocol recommended by the BACTERIOLOGICAL ANALYTICAL MANUAL/BAM-USDA (2014), with certain modifications. Twenty-five grams of each sample were weighed in a sterile plastic bag and 225 mL buffered peptone water (BPW, Difco, Franklin Lakes, New Jersey, USA) was added. The sample was then incubated at 36 °C for 24 h. After incubation, 0.1 mL and 1 mL of the BPW solutions was transferred to tubes containing 10 mL of Rappaport-Vassiliadis broth (RV, Difco) and 10 mL of tetrathionate broth (TT, Difco), respectively. RV and TT broths containing samples were incubated at 42 °C and 36 °C, for 24 h, respectively. Aliquots of both were then streaked onto plates containing bismuth sulfite agar (BS, Difco) and xylose lysine deoxycholate agar (XLD, Difco), and incubated at 36 °C for 24 h. Colonies that showed typical Salmonella spp. Characteristics (BS - brown, gray, or black colonies with a metallic sheen; XLD - pink colonies with or without black centers) were subjected to biochemical (lysine, H2S, urease, indole, methyl red, Voges-Proskauer, and citrate) and serological (polyvalent anti-Salmonella serum, Probac do Brasil) tests for confirmation. The isolates that were confirmed according to the biochemical and serological tests were subjected to PCR molecular confirmation according to protocols described by ALMEIDA et al. (2014). Results were expressed as either the presence or absence of Salmonella spp. in a 25 g sample.
Listeria monocytogenes
Listeria monocytogenes were detected according to PAGOTTO et al. (2001). After weighing 25 g of the sample, 225 mL Listeria Enrichment Broth (LEB, Difco) was added with subsequent incubation at 30 °C for 48 h. Then, a 0.1 mL aliquot of sample-containing LEB was transferred to a tube containing 10 mL Fraser broth, and incubated at 36 °C for 48 h. Aliquots of dark Fraser broth after esculin hydrolysis were streaked on plates containing Palcam Agar (Difco) and Oxford Agar (Difco), and then incubated at 36 °C for 24-48 h. To verify their purity, three to five colonies from Palcam and Oxford agars that showed characteristics of L. monocytogenes were seeded in Tryptic Soy Agar (Difco) with 0.6% Yeast Extract (TSA-YE), and incubated at 36 °C for 24-48 h. The characteristic isolates (1-3-mm diameter smooth convex white colonies) were then subjected to various tests, including catalase, fermentation of carbohydrates (dextrose, xylose, rhamnose, and mannitol), and the production of β-hemolysis in 5% sheep blood agar. Results were expressed as the presence or absence of L. monocytogenes in a 25 g sample. Isolates that were confirmed by biochemical tests were analyzed by PCR in accordance with extraction, amplification, and interpretation protocols described by DOUMITH et al. (2004).
Data analysis
Results of the bacterial quantification were submitted to the Shapiro-Wilk and Kolmogorov-Smirnov statistical tests to verify their normal distribution. Subsequently, the Kruskal Wallis and Mann-Whitney non-parametric tests were applied for statistical comparison among mean values of different types of samples (beef, chicken, pork, and fresh sausages) and their origins (butchers, supermarkets, and groceries). The Salmonella and L. monocytogenes results were expressed in frequencies and subjected to a chi-square test to verify the association among the presence of each pathogen, different types of retail samples and each retail market. In addition, results were compared with the Brazilian legislation being expressed in “compliance” and “noncompliance” to official parameters. The RDC 12 (BRASIL, 2001) was used as the Salmonella standard (Absence in 25 g). For the mesophilic aerobes, the IN 60 (BRASIL, 2019) was used as a basis; however, we emphasized that such legislation will enter into force only on December 23, 2020.
All statistical analyses were performed using the IBM® (Armonk, New York, USA) SPSS® Statistics version 20.0 statistical software, with a significance level of 0.05.
RESULTS AND DISCUSSION:
The mesophilic aerobe counts were higher in fresh sausages (p<0.05) than in the other products (Table 1). Samples from butcheries showed higher mesophile counts than the other types of establishments (p<0.05). Enterobacterial counts were higher in fresh sausages and beef (p<0.05) compared with chicken and pork. Butchers and grocery stores had higher counts than supermarkets (p<0.05).
The indicator microorganisms reflect the general microbiological quality of the products, demonstrating the hygienic sanitary conditions for production, handling, and storage in retail locations (SAINI et al., 2011). Our results showed average mesophilic counts that conform to the standards established by the Brazilian legislation (BRASIL, 2019). Regarding the enterobacteria analyses; although, there is no standard established protocol in the legislation for this group for meat and meat products, even low microorganism counts reinforce the need to evaluate sanitary conditions in places where these products are handled (SAINI et al., 2011).
Salmonella spp. was detected in 3.36% of samples and was present at the highest frequencies in beef (7.93%) and chicken (4.91%) (p<0.05; Table 2). Among the 18 commercial establishments evaluated, Salmonella spp. were found in seven locations (38.89 %), consisting of two butchers, three supermarkets, and two groceries. Listeria monocytogenes was reported in 13.86% of the samples analyzed, and at a particularly high frequency in fresh sausages (19.04%) compared with other types of meat (p<0.05). Commercial butchers and supermarkets exhibited the highest frequencies of L. monocytogenes (p<0.05), being isolated in 12 (66.67%) of 18 commercial establishments, including six supermarkets, five butcheries, and only one grocer.
The presence of the abovementioned pathogens highlights the need for constant health surveillance in these locations to prevent the spread of the pathogen during handling in food retail establishments. However, the lack of inspections in border regions means that pathogen-containing products carry contamination to the commercial environment. Due to failures in hygienic processes, the pathogens remain on surfaces, equipment and utensils for long periods. In this context, active health surveillance is the basis for maintaining an environment suitable for handling food.
In interior cities and border regions, the health surveillance services are linked to municipal health departments and they perform other functions in addition to on-site surveillance of commercial establishments. Thus, many of the inspection actions are reactive; that is, they are only triggered following a complaint from the consumer. For these reasons, in many Brazilian cities, especially those in the interior and in the border region, hygiene deficiencies in food establishments are routine and directly influence the health of the population in these places.
The Brazilian legislation that supports the surveillance at food-selling points established zero tolerance for S. Enteritidis, S. Typhimurium in meat products (BRASIL, 2019). Studies in Brazil have reported occurrence rates for these pathogens in meat cuts and fresh sausages at retail between 5.80% and 31.50% (BRIZIO et al., 2015; RISTORI et al. 2017; BIER et al., 2018; CAVALIN et al., 2018; PERIN et al., 2019). The grinding process used to make ground beef and the ground meat to use in fresh sausages can cause contamination through interactions with remnants from previous processes or dirt, due to hygiene failures (MOLLER et al., 2016). In our current study, a butchery ground beef sample tested positive for simultaneous contamination with Salmonella spp. and L. monocytogenes.
Besides its impact on the production chains for beef and pork, the spread of Salmonella spp. in the poultry chain is of great importance. This is because of epidemiological involvement in the chicken meat production system, and the spread thereof in the retail environment (CUNHA-NETO et al., 2019; PERIN et al., 2019). In this study, we observed Salmonella spp. In 4.91% of samples, which is consistent with previous research on the pathogen in Brazilian retail chicken cuts that demonstrated rates between 2.15% and 31.50% (PANZENHAGEN et al., 2016; RISTORI et al., 2017; FREITAS et al., 2019; PERIN et al., 2019).
The high occurrence of L. monocytogenes in meat products validates a public health concern (ESFA, 2018; CDC, 2019). The occurrence rates for this pathogen vary in meat products at retail, ranging from 8.16 - 48.70% (RISTORI et al., 2014; ELMALI et al., 2015; VALLIM et al., 2015; RODRIGUES et al., 2018; SILVA et al., 2020). According to these studies, this variation can be attributed to the category of meat products analyzed, sampling location, technological improvement employed by the commercial establishment, and geographical region of Brazil. However, processing and commercialization locations can influence the microbiological burden in products. Environments such as supermarkets and butchers, where the raw material for the processing of different meat cuts and other products is handled without following standardized norms for the processing, storage, and cleaning of facilities, can cause cross-contamination among products, handlers, and surfaces (BOECK et al., 2016; LEOTTA et al., 2016; SILVA et al., 2020).
As mentioned earlier, since a sanitary inspection in these establishments is flawed and often reactive, good food handling practices are not met, which facilitates the persistence of pathogens on surfaces and leads to food contamination, especially in establishments that sell directly to the consumer. Fresh sausages showed the highest L. monocytogenes frequencies (p < 0.05), corroborating studies by RISTORI et al. (2014), RODRIGUES et al. (2018), and SILVA et al. (2020), which investigated fresh sausages in different regions of Brazil. The process of preparing fresh sausages presents some critical points that deserve attention, mainly related to the process of grinding the raw material, use of condiments, and packaging in natural wrapping, in addition to the cross contamination of equipment and utensils that come into contact with the raw material. Thus, failures in some of these processes can lead to contamination of the final product (MOLLER et al., 2016).
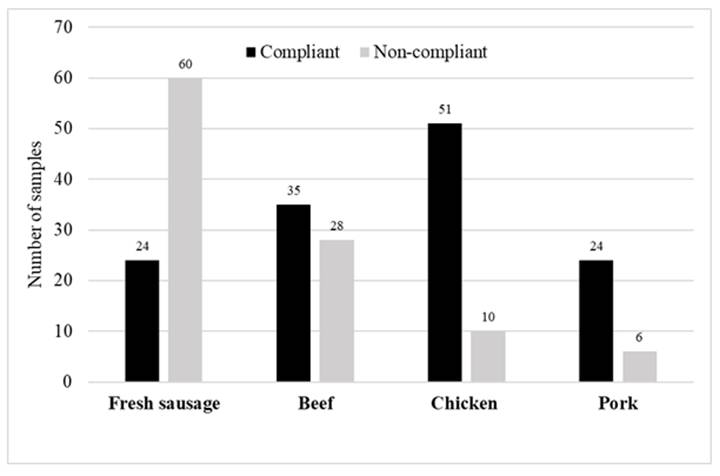
Of the 238 raw meat and fresh sausage samples analyzed, 104 did not comply with the microbiological parameters recommended by the Brazilian Regulation (BRASIL, 2001; 2019) (Figure 1). Concerning, 60 of fresh sausage samples breached the standards established for this product, with mesophilic counts above 1.0 × 106 log CFU/g (n = 52). These samples also contained L. monocytogenes (n = 8). This could be explained by the preparation processes for this product, which involve significant manipulation of the raw material and failures in the good manufacturing practice (GMP) processes (BOECK et al., 2016; LEOTTA et al., 2016). Additionally, the high noncompliance rate observed suggested that public agencies in these commercial locations must improve their vigilance, specifically by conducting programs to improve GMPs, and thus reduce consumer risk exposure. Fresh sausages are products closely linked to the culture of RS and are therefore present in the daily eating habits of the State’s population. Most of the fresh sausages consumed in the city of Uruguaiana are produced in the sales establishments themselves (supermarkets or butchers). Since they are not subjected to industrial inspection during production, process failures may go unchecked, which would lead to contamination by environmental pathogens.
× 106 log CFU/g for aerobic mesophiles and the absence of Salmonella spp. (BRASIL, 2001; BRASIL, 2019).
CONCLUSION:
A large number of samples of meat products analyzed in Uruguariana, RS reached the microbiological standards recommended by current legislation for indicator microorganisms. The presence of Salmonella spp. and L. monocytogenes in different samples can be the result of errors in the industrial process and lack of good manufacturing practices in industrial and commercial environments. This demonstrated the need for the sanitary education of handlers and consumers, improvement in self-control procedure programs by food industries and continuous monitoring by health bodies in both industrial and commercial establishments to reduce the exposure of consumers to products improper for consumption and the possibility of occur the foodborne diseases.
ACKNOWLEDGEMENTS
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 for providing a scholarship to Vanessa Mendonça Soares (PNPD CAPES Program). We thank the Universidade Federal do Pampa (UNIPAMPA) for providing a scholarship to Matheus Beltrame Padilha and Maria Eduarda de Moraes Guerra (Programa de Desenvolvimento à Pesquisa PDA 2018).
REFERENCES
-
ABUJAMRA, T., et al. Percepção dos consumidores em relação à segurança dos alimentos cárneos no município de Jataí - GO. Segurança Alimentar e Nutricional, v.24, n.1, p.9-16, 2017. Available from: <Available from: https://periodicos.sbu.unicamp.br/ojs/index.php/san/article/view/8648081 >. Accessed: Aug. 14, 2020.
» https://periodicos.sbu.unicamp.br/ojs/index.php/san/article/view/8648081 -
ALMEIDA, M. V.; et al.,Evaluation of target sequences for the polymerase chain reaction-based detection of Salmonella in artificially contaminated beef. Foodborne Pathogens and Disease, v.11, n.2, p.111-8, 2014. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/24102080/ >. Accessed: Nov. 5, 2020. doi: 10.1089/fpd.2013.1623.
» https://doi.org/10.1089/fpd.2013.1623.» https://pubmed.ncbi.nlm.nih.gov/24102080/ - BACTERIOLOGICAL ANALYTICAL MANUAL-USA. United States of America. Department of Health & Human Services U.S. Food and Drug Administration. Bacteriological Analytical Manual. Food Sampling and Preparation of Sample Homogenate. 2003.
- BACTERIOLOGICAL ANALYTICAL MANUAL-USA. United States of America. Department of Health & Human Services. U.S. Food and Drug Administration. Bacteriological Analytical Manual. Salmonella 2014.
-
BIER, D., et al. Survey of Salmonella spp. in beef meat for export at slaughterhouses in Brazil. Pesquisa Veterinária Brasileira, v.38, n.11, p.2037-2043. 2018. Available from: <Available from: https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-736X2018001102037 >. Accessed: Jun. 12, 2020. doi: 10.1590/1678-5150-pvb-5867.
» https://doi.org/10.1590/1678-5150-pvb-5867.» https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-736X2018001102037 -
BOECK, E., et al. Interplay between food safety climate, food safety management system and microbiological hygiene in farm butcheries and affiliated butcher shops. Food Control, v.65, p.78-91, 2016. Available from: <Available from: https://www.sciencedirect.com/science/article/abs/pii/S095671351630015 >. Accessed: Jun. 12, 2020. doi: 10.1016/j.foodcont.2016.01.014.
» https://doi.org/10.1016/j.foodcont.2016.01.014.» https://www.sciencedirect.com/science/article/abs/pii/S095671351630015 - BRASIL. Ministério da Saúde. Secretaria de Vigilância Sanitária. (Portaria nº 1.428, de 26 de novembro de 1993). Diário Oficial da União, Brasília, DF. 1993.
- BRASIL. Agência Nacional de Vigilância Sanitária. Ministério da Saúde. Regulamento Técnico sobre Padrões Microbiológicos para Alimentos. (Resolução RDC nº12, de 2 de janeiro de 2001). Diário Oficial da União , Brasília, DF. 2001.
-
BRASIL, Ministério da Saúde, Unidade de Vigilância das Doenças de Transmissão Hídrica e Alimentar. Doenças transmitidas por alimentos: causas, sintomas, tratamento e prevenção. Available from: <Available from: https://www.saude.gov.br/saude-de-a-z/doenca-diarreica-aguda >. 2020. Accessed: Jun. 12, 2020.
» https://www.saude.gov.br/saude-de-a-z/doenca-diarreica-aguda - BRASIL. Agência Nacional de Vigilância Sanitária. Ministério da Saúde. Estabelece as listas de padrões microbiológicos para alimentos. (Instrução Normativa nº 60, de 23 de dezembro de 2019). Diário Oficial da União , Brasília, DF. 2019.
-
BRIZIO, A. P. D. R., et al. Chilled broiler carcasses: A study on the prevalence of Salmonella, Listeria and Campylobacter International Food Research Journal, v.22, n.1, p.55, 2015. Available from: <Available from: http://www.ifrj.upm.edu.my/22%20(01)%202015/(8).pdf >. Accessed: Jun. 12, 2020.
» http://www.ifrj.upm.edu.my/22%20(01)%202015/(8).pdf -
CAVALIN, P. B. B., et al. Detection of Salmonella spp. and diarrheagenic Escherichia coli in fresh pork sausages. Semina: Ciências Agrárias, Londrina, v.39, n.4, p.1533-1546, 2018. Available from: <Available from: http://www.uel.br/revistas/uel//index.php/semagrarias/article/view/32068 >. Accessed: Jun. 12, 2020. doi: 10.5433/1679-0359.2018v39n4p1533.
» https://doi.org/10.5433/1679-0359.2018v39n4p1533.» http://www.uel.br/revistas/uel//index.php/semagrarias/article/view/32068 -
CDC, Centers for Disease Control and Prevention. Foodborne Outbreak Tracking and Reporting (FOOD Tool). Availabe from: < Availabe from: https://wwwn.cdc.gov/norsdashboard/ >. 2019. Accessed: Jun. 12, 2020.
» https://wwwn.cdc.gov/norsdashboard/ -
CUNHA-NETO, A., et al. Occurrence and antimicrobial resistance profile of Salmonella isolated from native fish slaughtered and commercialised in Brazil. Journal of Food Safety and Food Quality, v.70, n.4, p.91-124, 2019. Available from: <Available from: https://journal-food-safety.de/Article-Details/285 >. Accessed: Jun. 12, 2020.
» https://journal-food-safety.de/Article-Details/285 -
DEMAÎTRE, N., et al. Occurrence, distribution and diversity of Listeria monocytogenes contamination on beef and pig carcasses after slaughter. Meat Science, v.108177, p.108177, 2020. Available from: <Available from: https://www.sciencedirect.com/science/article/abs/pii/S0309174020302011 >. Accessed: Jun. 12, 2020. doi: 10.1016/j.meatsci.2020.108177.
» https://doi.org/10.1016/j.meatsci.2020.108177.» https://www.sciencedirect.com/science/article/abs/pii/S0309174020302011 -
DOUMITH, M et al.,Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. Journal of Clinical Microbiology, v.42, n.8, p.3819-3822, 2004. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC497638/ >. Accessed: Nov. 5, 2020. doi: 10.1128/JCM.42.8.3819-3822.2004.
» https://doi.org/10.1128/JCM.42.8.3819-3822.2004.» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC497638/ -
EFSA, European Food Safety Authority. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA Journal, v.16, n.12, p.1-262, 2018. Available from: <Available from: https://www.efsa.europa.eu/en/efsajournal/pub/5500 >. Accessed: Jun. 12, 2020. doi: 10.2903/j.efsa.2018.5500.
» https://doi.org/10.2903/j.efsa.2018.5500.» https://www.efsa.europa.eu/en/efsajournal/pub/5500 -
ELMALI, M., et al. Prevalence of Listeria monocytogenes in poultry meat. Ciência e Tecnologia de Alimentos, v.35, n.4, p.672-675, 2015. Available from: <Available from: https://www.scielo.br/pdf/cta/v35n4/0101-2061-cta-1678-457X6808.pdf >. Accessed: Jun. 12, 2020. doi: 10.1590/1678-457X.6808.
» https://doi.org/10.1590/1678-457X.6808.» https://www.scielo.br/pdf/cta/v35n4/0101-2061-cta-1678-457X6808.pdf -
FANALLI, S. L. Perfil de consumo e percepção dos consumidores de carne: consequências sobre a saúde pública. Revista Científica de Medicina Veterinária, v.31, 2018. Available from: <Available from: http://faef.revista.inf.br/imagens_arquivos/arquivos_destaque/7YgU5DLnagIDsVr_2018-9-19-8-41-24.pdf >. Accessed: Aug. 14, 2020.
» http://faef.revista.inf.br/imagens_arquivos/arquivos_destaque/7YgU5DLnagIDsVr_2018-9-19-8-41-24.pdf -
FREITAS, F., et al. Microbiological evaluation of thigh and drumstick chicken sold in bulk in Sinop-MT. Ciência Animal Brasileira, v.20, 2019. Available from: <Available from: https://www.scielo.br/pdf/cab/v20/1809-6891-cab-20-e50116.pdf >. Accessed: Jun. 12, 2020. doi: 10.1590/1809-6891v20e-50116.
» https://doi.org/10.1590/1809-6891v20e-50116.» https://www.scielo.br/pdf/cab/v20/1809-6891-cab-20-e50116.pdf -
GONÇALVES-TENÓRIO, A., et al. Prevalence of pathogens in poultry Meat: A Meta-Analysis of European Published Surveys. Foods, v.7, n.5, p.61-69, 2018. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5977089/pdf/foods-07-00069.pdf >. Accessed: Aug. 14, 2020. doi: 10.3390/foods7050069.
» https://doi.org/10.3390/foods7050069.» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5977089/pdf/foods-07-00069.pdf -
JARVIS, N. A., et al. An overview of Salmonella thermal destruction during food processing and preparation. Food Control, v.68, p.280-290, 2016. Available from: <Available from: https://www.sciencedirect.com/science/article/abs/pii/S095671351630175X >. Accessed: Jun. 12, 2020. doi: 10.1016/j.foodcont.2016.04.006.
» https://doi.org/10.1016/j.foodcont.2016.04.006» https://www.sciencedirect.com/science/article/abs/pii/S095671351630175X - LAWRIE, R. A. Ciência da carne. 6ed. Porto Alegre: Artmed, p.384. 2005.
-
LEOTTA, G. A., et al. Comprehensive evaluation and implementation of improvement actions in butcher shops. PLoS One, v.11, n.9, p.e0162635, 2016. Available from: <Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0162635 >. Accessed: Jun. 12, 2020. doi: 10.1371/journal.pone.0162635.
» https://doi.org/10.1371/journal.pone.0162635.» https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0162635 -
LOPES, M. A., et al. Fatores associados a disposição de consumidores em adquirir carne bovina com certificação de origem na cidade do Rio de Janeiro, Brasil. Brazilian Journal of Veterinary Medicine, v.39, n.2, p.100-110, 2017. Available from: <Available from: http://rbmv.org/index.php/BJVM/article/download/913/756 > Accessed: Aug. 14, 2020. doi: 10.29374/2527-2179.bjvm027117.
» https://doi.org/10.29374/2527-2179.bjvm027117.» http://rbmv.org/index.php/BJVM/article/download/913/756 -
MOHAMED, R. I., et al. Virulence and antimicrobial susceptibility profile of Listeria monocytogenes isolated from frozen vegetables available in the Egyptian market. African Journal of Microbiology Research, v.12, n.9, p.218-224, 2018. Available from: <Available from: https://academicjournals.org/journal/AJMR/article-full-text-pdf/0C8BBCD56217 >. Accessed: Aug. 14, 2020. doi: 10.5897/AJMR2018.8794.
» https://doi.org/10.5897/AJMR2018.8794.» https://academicjournals.org/journal/AJMR/article-full-text-pdf/0C8BBCD56217 -
MOLLER, C. O. A., et al. Evaluation of a cross contamination model describing transfer of Salmonella spp. and Listeria monocytogenes during grinding of pork and beef. International Journal of Food Microbiology, v.226, p.42-52, 2016. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/27035678/ >. Accessed: Jun. 12, 2020. doi: 10.1016/j.ijfoodmicro.2016.03.016.
» https://doi.org/10.1016/j.ijfoodmicro.2016.03.016.» https://pubmed.ncbi.nlm.nih.gov/27035678/ - PAGOTTO, F., et al. MFHPB-30. Isolation of Listeria monocytogenes and other Listeria spp. from foods and environmental samples. In: Compendium of Analytical Methods. 2001.
-
PANZENHAGEN, P. H. N., et al. Prevalence and fluoroquinolones resistance of Campylobacter and Salmonella isolates from poultry carcasses in Rio de Janeiro, Brazil. Food Control , v.61, p.243-247, 2016. Available from: <Available from: https://www.sciencedirect.com/science/article/abs/pii/S0956713515302243 >. Accessed: Jun. 12, 2020. doi: 10.1016/j.foodcont.2015.10.002.
» https://doi.org/10.1016/j.foodcont.2015.10.002.» https://www.sciencedirect.com/science/article/abs/pii/S0956713515302243 -
PEREIRA, J. G., et al. Profile of the illegal import of products of animal origin to Brazilian cities at the border with Argentina and Uruguay. Journal of Food Protection, v.80, n.10, p.1605-1612, 2017. Available from: <Available from: https://meridian.allenpress.com/jfp/article-abstract/80/10/1605/199969/Profile-of-the-Illegal-Import-of-Products-of?redirectedFrom=fulltext >. Accessed: Aug. 14, 2020. doi: 10.4315/0362-028X.JFP-17-123.
» https://doi.org/10.4315/0362-028X.JFP-17-123.» https://meridian.allenpress.com/jfp/article-abstract/80/10/1605/199969/Profile-of-the-Illegal-Import-of-Products-of?redirectedFrom=fulltext -
PERIN, A. P., et al. Occurrence, quantification, pulse types, and antimicrobial susceptibility of Salmonella sp. isolated from chicken meat in the state of Paraná, Brazil. Brazilian Journal of Microbiology, p.1-11, 2019. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/31782062/ >. Accessed: Jun. 12, 2020. doi: 10.1007/s42770-019-00188-x.
» https://doi.org/10.1007/s42770-019-00188-x.» https://pubmed.ncbi.nlm.nih.gov/31782062/ -
RESENDE, A. H. V.; SANTOS, R. A. Principais dificuldades enfrentadas no setor de vigilância sanitária municipal. Anais eletrônicos da I CIEGESI, 2012. Available from: <Available from: https://www.anais.ueg.br/index.php/ciegesi/article/view/1158/869 >. Accessed: Aug. 14, 2020.
» https://www.anais.ueg.br/index.php/ciegesi/article/view/1158/869 -
RISTORI, C. A., et al. Prevalence and populations of Listeria monocytogenes in meat products retailed in São Paulo, Brazil. Foodborne Pathogens and Disease , v.11, n.12, p.969-973, 2014. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/25407460/ >. Accessed: Jun. 12, 2020. doi: 10.1089/fpd.2014.1809.
» https://doi.org/10.1089/fpd.2014.1809.» https://pubmed.ncbi.nlm.nih.gov/25407460/ -
RISTORI, C. A., et al. Assessment of consumer exposure to Salmonella spp., Campylobacter spp., and Shiga Toxin-Producing Escherichia coli in meat products at retail in the city of São Paulo, Brazil. Foodborne Pathogens and Disease , v.14, n.8, p.447-453, 2017. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/28475359/ >. Accessed: Jun. 12, 2020. doi: 10.1089/fpd.2016.2270.
» https://doi.org/10.1089/fpd.2016.2270.» https://pubmed.ncbi.nlm.nih.gov/28475359/ -
RODRIGUES, C. S., et al. Listeria monocytogenes contamination in industrial sausages. Brazilian Journal of Veterinary Medicine , v.40, p.e009118-e009118, 2018. Available from: <Available from: http://rbmv.org/index.php/BJVM/article/view/91 >. Accessed: Jun. 12, 2020. doi: 10.29374/2527-2179.bjvm009118.
» https://doi.org/10.29374/2527-2179.bjvm009118.» http://rbmv.org/index.php/BJVM/article/view/91 -
SAINI, P. K., et al. Indicator organisms in meat and poultry slaughter operations: their potential use in process control and the role of emerging technologies. Journal of Food Protection , v.74, n.8, p.1387-1394, 2011. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/21819672/ >. Accessed: Jun. 12, 2020. doi: 10.4315/0362-028X.JFP-10-433.
» https://doi.org/10.4315/0362-028X.JFP-10-433.» https://pubmed.ncbi.nlm.nih.gov/21819672/ -
SILVA, D. A. L., et al. Listeria monocytogenes From Farm to Fork in a Brazilian Pork Production Chain. Journal of Food Protection , v.83, n.3, p.485-490, 2020. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/32065647/ >. Accessed: Jun. 12, 2020. doi: 10.4315/0362-028X.JFP-19-379.
» https://doi.org/10.4315/0362-028X.JFP-19-379.» https://pubmed.ncbi.nlm.nih.gov/32065647/ -
VALLIM, D. C., et al. Twenty years of Listeria in Brazil: Occurrence of Listeria species and Listeria monocytogenes serovars in food samples in Brazil between 1990 and 2012. BioMed Research International, 2015. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/26539507/ >. Accessed: Jun. 12, 2020. doi: 10.1155/2015/540204.
» https://doi.org/10.1155/2015/540204.» https://pubmed.ncbi.nlm.nih.gov/26539507/ -
ZENG, Y. B., et al. Prevalence and Antimicrobial Resistance of Salmonella in Pork, Chicken, and Duck from Retail Markets of China. Foodborne Pathogens and Disease , v.16, n.5, p.1-7, 2019. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/31013442/ >. Accessed: Jun. 12, 2020. doi: 10.1089/fpd.2018.2510
» https://doi.org/10.1089/fpd.2018.2510» https://pubmed.ncbi.nlm.nih.gov/31013442/
Publication Dates
-
Publication in this collection
26 Mar 2021 -
Date of issue
2021
History
-
Received
15 June 2020 -
Accepted
06 Nov 2020 -
Reviewed
04 Jan 2020

 Identification of Salmonella spp., Listeria monocytogenes, and indicator microorganisms in commercialized raw meats and fresh sausages from Uruguaiana, Rio Grande do Sul, Brazil
Identification of Salmonella spp., Listeria monocytogenes, and indicator microorganisms in commercialized raw meats and fresh sausages from Uruguaiana, Rio Grande do Sul, Brazil