Abstracts
The objective of this study was to evaluate the interface formed between the poli (viniilidene) fluoride (PVDF-piezoelectric and non-piezoelectric) and cap rats' bone tissue. Twenty tubes of PVDF [P (VDF-TrFE)] piezoelectric, (d3h = 2,5 pC/N and capacitance 800 pF/m), and twenty tubes of non-piezoelectric PVDF were implanted in the intercondilian notch of the left femur of 40 rats. The animals of both groups were subdivided in four subgroups, followed up for 7 days, 3, 6 and 12 weeks. The interface found between bone and tubes was studied by conventional optical microscopy (MOC) (n=28) and by backscattered electronic scanning microscopy (MEV) (n=12). Bone tissue growth was observed inside the tubes of piezoelectric PVDF followed up during 12 weeks, both by MOC and by MEV backscattering. The results indicate that the piezoelectric effect had an important role in the new bone tissue formation inside the piezoelectric tubes. Probably, that bone formation was a result from the electrets effect or from micro deformations produced in the piezoelectric tubes, due to the intra-articular pressure variation on the knee movement during gait.
Piezoelectric effect; Bone growth; Artificial implants
O objetivo deste estudo foi analisar a interface formada entre o polifluoreto de vinilideno (PVDF - piezelétrico e não piezelétrico) e o tecido ósseo do rato. Foram implantados em 40 ratos, na região intercondiliana do fêmur esquerdo, vinte tubos de PVDF [P(VDF-TrFE)] piezelétricos, (d3h = 2,5 pC/N e capacitância 800 pF/m), e vinte tubos de PVDF não piezelétricos. Os animais de ambos os grupos foram subdivididos em quatro subgrupos, seguidos por 7 dias, 3, 6 e 12 semanas. A interface formada pelos tubos com o tecido ósseo foi estudada por microscopia óptica convencional (MOC) (n=28) e pela microscopia eletrônica de varredura (MEV) por retroespalhamento (n=12). No interior dos tubos de PVDF piezelétricos seguidos por 12 semanas foi constatado, tanto pela MOC como pela MEV por retroespalhamento, crescimento de tecido ósseo. Os resultados indicam que a piezeletricidade teve papel importante na neoformação do tecido ósseo no interior dos tubos piezelétricos. Provavelmente, essa formação óssea foi decorrente ou do efeito eletreto, ou das microdeformações produzidas nos tubos piezelétricos, devido à variação da pressão intra articular do joelho durante a marcha.
Piezeletricidade; Crescimento ósseo; Implantes artificiais
ORIGINAL ARTICLE
Analysis of the interface formed among the poli (viniilidene) fluoride (piezoelectric and non-piezoelectric) and the bone tissue of rats
Bianca CallegariI; Dr William Dias BelangeroII
IPost-Graduate in Surgery, area of Experimental Research
IIManager of laboratory
Correspondence Correspondence to Rua Emílio Ribas no. 800, apto 1, Cambuí 13025-041 Campinas/SP E-mail: belanger@fcm.unicamp.br
SUMMARY
The objective of this study was to evaluate the interface formed between the poli (viniilidene) fluoride (PVDF-piezoelectric and non-piezoelectric) and cap rats' bone tissue. Twenty tubes of PVDF [P (VDF-TrFE)] piezoelectric, (d3h = 2,5 pC/N and capacitance 800 pF/m), and twenty tubes of non-piezoelectric PVDF were implanted in the intercondilian notch of the left femur of 40 rats. The animals of both groups were subdivided in four subgroups, followed up for 7 days, 3, 6 and 12 weeks. The interface found between bone and tubes was studied by conventional optical microscopy (MOC) (n=28) and by backscattered electronic scanning microscopy (MEV) (n=12). Bone tissue growth was observed inside the tubes of piezoelectric PVDF followed up during 12 weeks, both by MOC and by MEV backscattering.
The results indicate that the piezoelectric effect had an important role in the new bone tissue formation inside the piezoelectric tubes. Probably, that bone formation was a result from the electrets effect or from micro deformations produced in the piezoelectric tubes, due to the intra-articular pressure variation on the knee movement during gait.
Key words: Piezoelectric effect, Bone growth, Artificial implants
INTRODUCTION
The piezoelectricity is a property present in the materials in their crystalline state. Biological material rich in collagen, such as bone and tendon, may show this property which, in a certain way, would be responsible for the adaptation of these tissues to the mechanical demands that the outside environment imposes(3).
The effect of the piezoelectricity was studied in our area by Köberle(9) in his free-teaching thesis. Yasuda(15) also studied in rabbits the effect of the piezoelectricity applying Teflon (electrically polarized and non-polarized) on the surface of the femur and rolled up around this bone. He observed that below the non-polarized Teflon there was no bone growth, while around the polarized Teflon there was a formation of bone callus, mainly in those in that Teflon had been rolled up around of the femur.
More recently, Jianqing et al.(5) compared the effects of non-piezoelectric and piezoelectric ceramic on the new formation of bone in the jaws of dogs. Bone growth was observed around of the piezoelectric implants after one week, while after two weeks there still was no new formation of bone around of the no piezoelectric implants.
Taking into account that there are indications that the piezoelectricity exercises influence on bone growth, the use of materials with this characteristic as a bone implant seems to be promising.
The poli (viniilidene) fluoride, also known as viniilidene fluoride (PVDF), is a semi-crystalline polymer (50% crystalline, 50% amorphous), whose basic unit is the F2C=CH2. PVDF can be polarized because it presents that chemical composition. The atoms of Hydrogen (H) are charged positively, while the fluorine (F) ones are charged negatively, in relation to the atoms of carbon (C). The PVDF may be found under different crystalline forms; although it is not a polar form, alpha is the most common of these forms. On the other hand, the b phase can be polarized, and thus presents, the piezoelectric properties, normally obtained from the alpha form, by mechanical stretching(13).
From the clinical standpoint, PVDF is a polymer used as suture material in vascular surgeries. It also shows a superior biological and mechanic performance in relation to the polypropylene because it is more biocompatible, and because it preserves its mechanical resistance up to 92,5% after 9 years of the implant, while polypropylene loses 46% of this resistance during the same period(8,10).
Notwithstanding these characteristics, there are few studies that evaluated the potentiality of this material for the production of orthopedic implants. Within our area, Paschoal(12) demonstrated that polarized PVDF membranes, when implanted between the cortical lateral of the femur of a rabbit and one metallic plate, it stimulated the bone growth. Due to these theoretical considerations, it is possible to study the effects of piezoelectric PVDF implants on the bone tissue.
MATERIAL AND METHOD
Twenty piezoelectric PVDF [P(VDF-TrFE)] tubes and 20 tubes of the same material, but non-piezoelectric, were made available by the Physics, Chemistry and Biology Department of the Sciences and Technology College of the UNESP.
The piezoelectric tubes with a d3h = 2,5 pC/N coefficient and a capacitance of 800 pF/m were cut along the length, and were 5 mm long, with an external diameter of 1,9 mm and internal diameter of 0,8 mm®1.
The non-piezoelectric tubes were obtained from the piezoelectric tubes blended at a temperature of 150º C, with 5 mm length, 2 mm external diameter and 0,7 mm internal diameter.
Experimentation animals
Forty male Rattus Novergicus of the Wistar variety were used, supplied by Central Biotery of the UNICAMP. The procedures were authorized by the Ethics Committee for Animal Experimentation of the Institute of Biology of UNICAMP.
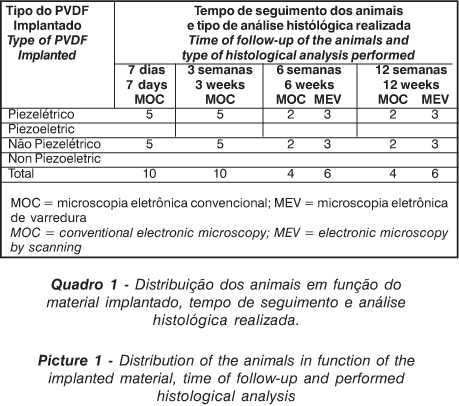
Twenty piezoelectric tubes were implanted in twenty animals, and 20 non-piezoelectric tubes were implanted in other 20 animals, all in the intercondilian area of the femur. In each group, the 20 animals were divided into four subgroups, followed during 7 days (5 animals), 3 and 6 weeks and 12 weeks (5 animals).
Surgical procedure
After the preoperative fast, the animals were anesthetized with intravenous sodium Pentobarbital®2 in the dosage of 50mg/Kg. The intercondilian area was exposed by a parapatelar lateral access. The tubes were implanted in the intercondilian area of the femur of the left paw of the mice, using bears with diameters identical to the diameters of the tubes with a low rotation drill®(3). The depth of the hole was of 5 mm, in such a way as to maintain the surface of the tube even with the articular surface.
After the termination of the follow-up period, the animals were sacrificed through an overdose of sodium Pentobarbital, and the third distal of the femurs of the left paw were removed. All of the samples were fixed in formaldehyde 10% solution of the tamponade for 24 hours, except for 3 animals of each group with a follow-up period of 6 and 12 weeks, that remained in an alcoholic solution at 70% for 24 hours.
After the fixation, all the samples removed from the groups with a follow-up period of 7 days and 3 weeks were decalcified in a nitric acid solution at 3% during 3 days, and were bivalved for the removal of the tubes, with a minimum lesion to the samples. The samples, without the tubes, were prepared for the histological analysis and the sections obtained were tinted with Hematoxylin Eosin and Masson Trichomycous.
Three samples removed from each one of the groups with a follow-up period of 6 and 12 weeks, were incrusted with acrylic resin®(4) and submitted to a retro spreading Electronic Microscopy of Scanning (EMS) for the evaluation of the interface formed between the polymer and the bone (Picture 1).
RESULTS
Macroscopic analysis
The cicatrisation of the skin and of the soft tissues was from the seventh day on, and it was observed that the animal strolled without restrictions. The articular surface of the intercondilian area was observed during the sacrifice of the animals and it was verified that that surface was more regular in the animals in that had been implanted with the piezoelectric tubes (Figure 1).
The histological analysis of the samples with 7 days, 3, 6 and 12 weeks (n=14) showed that there was no formation of a fibrous capsule around of the implants in both groups. The removal of the tubes of polarized PVDF from the bone tissue was more difficult, because they were more adhered.
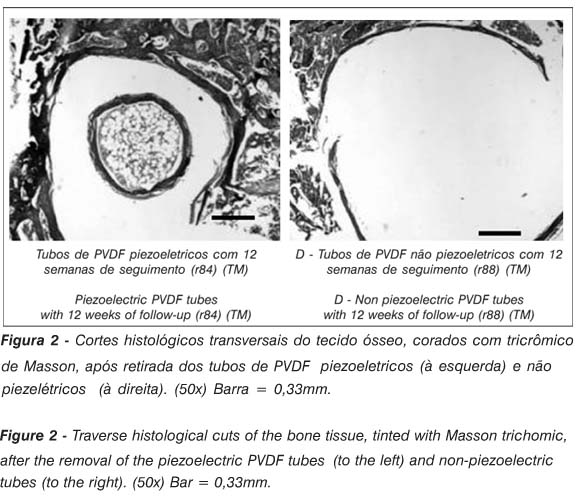
The formation of a halo, involving the whole internal and external surface of the tubes, was observed on the laminae obtained from the group of 12 weeks (n=2), with the aspect of bone tissue (Figure 2). No bone tissue growth was observed in the animals implanted with the non-piezoelectric PVDF tubes.
The electronic Microscopy scanning by retrospread beam showed that the interface formed between the tubes and the bone was constituted essentially from bone tissue. Inside the piezoelectric PVDF tubes followed up for 12 weeks (n = 3) there was a growth of bone tissue, while, during the same period, the growth of that tissue was not observed inside of the non-piezoelectric tubes (Figure 3).
DISCUSSION
Some findings from the results analyzed must be underlined: the more uniform and regular aspect of the articular surface of the intercondilian area with the piezoelectric tubes; the difficulty of removing the piezoelectric tubes from the bone bed and, finally, the discovery of bone tissue just inside the piezoelectric tubes. These findings suggest that the piezoelectricity must have influenced that behavior, since the piezoelectric tubes, as much as the non-piezoelectric tubes, had the same origin, chemical composition and superficial linings.
The manner in which the bone tissue interprets the piezoelectric stimulus and transforms it into biological activity is still not clearly explained in the literature, so much as for the difficulty of mimetizing the real environmental conditions of the bone tissue, as for the fact that the knowledge of the intra and extracellular communication mechanisms is still a relatively recent subject. The biology studies during the last decades have been focused on the knowledge of the extracellular signaling molecules, because they make the cells capable of responding to certain incentives. These signaling molecules link to receptor proteins of the cellular surface and later to the intracellular signaling proteins, which direct the signal to appropriate regions of the cell. In the end of this process are the target proteins, that are the final effectors and induce the modification in the cellular behavior(1).
Experimental studies have been trying to define which is the molecule or system that translates the language of the physical stimulus into biological language, but until now the most current data on the subject were defined based on experimental studies of cell cultures that, although very useful for the explanation of isolated phenomena, cannot be extrapolated for what happens with the tissue within the body as a whole. According to Roy et al(14), the studies performed during the last 10 years only began to elucidate the aspects of the transmembrane signaling , and the genical expression of the bone tissue in response to biophysical stimuli, as for instance, the alteration in the flow of the canaliculi that link the osteocytes.
The mechanical deformation at physiologic levels can create different hydrostatic pressure gradients in the interstitial fluid of the caniculi of the bone tissue, that are captured by the cellular membrane of the osteocyte, resulting in the increase of its metabolic activity, suggesting that the osteocyte is the responsible cell for the transduction process from the mechanical to the biological stimulus. On the other hand, the in vivo or in vitro studies have also been showing a rather uniform response of the bone tissue regarding the electric stimuli that increase the production and remodeling of the bone. In addition, the therapeutic use of those techniques, including the ultrasound, electric and electromagnetic stimuli have been leading to good clinical results, although they are still developed in an empiric manner, without very defined dose-effect schemes, mainly due to the lack of a better understanding of the interactions between these techniques with the cell membrane(2).
According to information received from supplier of the piezoelectric tubes, those tubes were polarized with the bombardment of negative charges, and thus the outside wall is charged positively and the inside wall negatively, since the bombardment attracted the positive charges to the outside wall, driving in the inside of the material the dipoles and thus making the negative charges concentrated on the inside wall.
Under those conditions, if that tube suffered any compression, the positive charges would be generated on one of the faces and the negative charges, on the other face. In case it suffered a stretching effort, the polarity of the charges would be inverted. In this manner the negative charges could be generated on the outside surface, as well as on the inside surface of the tube, stimulating the growth inside and outside of the tube.
The type and the magnitude of the mechanical deformation that the tubes underwent in this study can be questioned because, as they are implanted in distal metaphysical area of the femurs, it was expected that they didn't suffer any deformations. How, then, can the piezoelectric effect be explained in this study? One of the possible explanations takes into account that the piezoelectric PVDF tube would work as an electret (or capacitor), that it stimulate the growth of the bone tissue by itself, in a similar way as the finding by Yasuda(15) with the polarized Teflon. In favor of that hypothesis is the fact that the bone growth was observed only inside the piezoelectric tubes, where the negative charges are concentrated. Therefore, it can be supposed that even after the manipulation and implantation of the tubes the inside surface remained charged, with the prevalence of negative charges that knowingly stimulate the bone growth(2,9).
The other possible explanation takes into consideration the fact that, as they were implanted in the intercondilian area of the femur, and as this is an intra-articular area, the tubes initiated a micro deformation process, due to the change of the intra-articular pressure gradient, generated by the movement of the articulation, and during the gait. Those micro deformations must have induced, due to the effect of the piezoelectricity, the formation of electric currents on the inside surface of the tubes, that were responsible for the growth of bone tissue, as suggested by some authors(4,6,7,11).
In spite that the results of this study indicate that there was a positive effect of the piezoelectric PVDF regarding the new formation of bone, new investigations are necessary to better quantify that phenomenon-and, from the clinical point of view, there is the need to evaluate its applicability.
It can be concluded that in the interface formed among the PVDF tube (piezoelectric and non-piezoelectric) with the bone tissue there was no growth of fibrous tissue, and that inside of the piezoelectric tubes there was a formation of bone tissue, including a trabecular arrangement.
REFERÊNCIAS BIBLIOGRÁFICAS
Trabalho recebido em 15/04/2004.
Aprovado em 20/06/2004.
Work performed at the Laboratory of Investigation in Orthopedic Materials (LABIMO)-Department of Orthopedics and Traumatology-University of Medical Sciences - UNICAMP
- 1. ALBERTS B, JOHNSON A, LAWIS J, REFF M, ROBERTS K, WALTER P. Chapter 15. In: Molecular biology of the cell. 4Ş. edição. EUA: Galan & Science, 2002. 831-906.
- 2. BRIGHTON CT, WANG W, SELDES R, ZHANG G, POLLACK SR. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am, 83-A(10):1514-1523, 2001.
- 3. FROST HM. Orthopaedic Biomechanics. Illinois (EUA), Charles C. Thomas (publisher), 1973, 652p, volume 5.
- 4. HAMAMOTO N, HAMAMOTO Y, NAKAJIMA T, OZAWA H. Histological, histocytochemical and ultrastructural study on the effects of surface charge on bone formation in the rabbit mandible. Arch Oral Biol, 40(2): 97-106, 1995.
- 5. JIANQING F, HUIPIN Y, XINGDONG Z. Promotion of osteogenesis by a piezoelectric biological ceramic. Biomaterials,18: 1531-1534, 1997.
- 6. JONES SJ, BOYDE A. The migration of osteoblasts. Cell Tissue Research. 184: 179-193, 1977.
- 7. KIZUKI T, OHGAKI M, KATSURA M, NAKAMURA S, HASHIMOTO K, TODA Y, UDAGAWA S, YAMASHITA K. Effect of bone-like layer growth from culture medium on adherence of osteoblast-like cells. Biomaterials,24(6): 941-947, 2003.
- 8. KLINGE U, KLOSTERHALFEN B, OTTINGER AP, JUNGE K, SCHUMPELICK V. PVDF as a new polymer for the construction of surgical meshes. Biomaterials, 23: 3487-3493, 2002.
- 9. KÖBERLE G. Estudos físicos e biológicos em tecido ósseo. Ribeirão Preto — São Paulo, 1974. (Tese - Livre docência - Faculdade de Medicina de Ribeirão Preto)
- 10. LAROCHE G, MAROIS Y, SCHWARZ E, GUIDOIN R, KING M, PARIS E, DOUVILLE Y. Polyvinylidene fluoride (PVDF) as a biomaterial: from polymeric raw material to monofilament vascular suture. J Biomed Mat Res. 29: 1525-1536, 1995.
- 11. NAKAMURA S, KOBAYASHI T, YAMASHITA K. Extended bioactivity in the proximity of hydroxyapatite ceramic surfaces induced by polarization charges. J Biomedical Mat Res. 61(4): 593-9, 2002.
- 12. PASCHOAL AL. Estudo da viabilidade de aplicação do polímero piezelétrico fluoreto de polivinilideno (PVDF) entre osso cortical e placa de osteossíntese para estimulação de crescimento ósseo. São Carlos/SP. 2003 (Tese — Doutorado - Universidade de São Paulo).
- 13. RIBEIRO PAMF. Influência da umidade nas propriedades elétricas do Fluoreto de Polivinilideno. Lisboa: Universidade Nova de Lisboa — Faculdade de Ciências e Tecnologia, 1994.
- 14. ROY AK, BOYAN BD, CIOMBOR DB, SCHWARTZ Z, SIMON B. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Rel Res. 1(419): 30-37, 2004.
- 15. YASUDA I. Electrical callus and callus formation by electret. Clin Orthop Rel Res, 124: 53-56, 1977.
Publication Dates
-
Publication in this collection
16 Nov 2004 -
Date of issue
Sept 2004
History
-
Received
15 Apr 2004 -
Accepted
20 June 2004